Testosterone Deficiency Promotes Hypercholesteremia and Attenuates Cholesterol Liver Uptake via AR/PCSK9/LDLR Pathways
- PMID: 35599686
- PMCID: PMC9122719
- DOI: 10.1155/2022/7989751
Testosterone Deficiency Promotes Hypercholesteremia and Attenuates Cholesterol Liver Uptake via AR/PCSK9/LDLR Pathways
Abstract
Background: Testosterone deficiency is reportedly correlated with an elevation of cholesterol in plasma, but the mechanism remains unclear. Our objective was to investigate the effects of testosterone deficiency on cholesterol metabolism and the corresponding molecular changes in vivo and in vitro.
Methods: SD rats were randomized into three groups: sham-operated (SHAM), subtotal orchiectomized (SO), and orchiectomized (ORX) and fed for 8 weeks. HepG2 cells were cultured with medium containing testosterone with the final concentrations of 0, 10, 30, and 300 nM. Method of isotope tracing and fluorescence labelling was adopted to investigate cholesterol metabolism. Several key molecules of cholesterol metabolism were also analyzed.
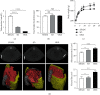
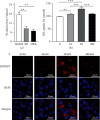
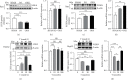
Results: SO and ORX rats displayed dysfunctional liver uptake of cholesterol. HepG2 cells incubated with testosterone of lower and excessive level exhibited reduced capacity of cholesterol uptake. Further investigation revealed that lack of testosterone induced increased proprotein convertase subtilisin/kexin type 9 (PCSK9) and decreased low-density lipoprotein receptor (LDLR) both in vivo and in vitro. Moreover, the androgen receptor (AR) antagonist flutamide mimicked the effects of testosterone deficiency on PCSK9 and LDLR indicating the role of AR as a mediator in triggering attenuating liver cholesterol uptake in which testosterone instead of dihydrotestosterone (DHT) is the major functional form of androgen.
Conclusion: Testosterone deficiency attenuated cholesterol liver uptake mediated by the PCSK9-LDLR pathway, in which AR and testosterone without transforming to DHT play important roles.
Copyright © 2022 Yu Yuefeng et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
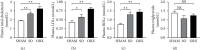

Similar articles
-
Cyclase-associated protein 1 is a binding partner of proprotein convertase subtilisin/kexin type-9 and is required for the degradation of low-density lipoprotein receptors by proprotein convertase subtilisin/kexin type-9.Eur Heart J. 2020 Jan 7;41(2):239-252. doi: 10.1093/eurheartj/ehz566. Eur Heart J. 2020. PMID: 31419281 Free PMC article.
-
Effect of testosterone deficiency on cholesterol metabolism in pigs fed a high-fat and high-cholesterol diet.Lipids Health Dis. 2015 Mar 7;14:18. doi: 10.1186/s12944-015-0014-5. Lipids Health Dis. 2015. PMID: 25889601 Free PMC article.
-
Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration.Hepatology. 2008 Aug;48(2):646-54. doi: 10.1002/hep.22354. Hepatology. 2008. PMID: 18666258
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
-
Proprotein Convertase Subtilisin/Kexin-Type 9 and Lipid Metabolism.Adv Exp Med Biol. 2020;1276:137-156. doi: 10.1007/978-981-15-6082-8_9. Adv Exp Med Biol. 2020. PMID: 32705598 Review.
Cited by
-
Cholesterol-metabolism, plant sterols, and long-term cognitive decline in older people - Effects of sex and APOEe4.iScience. 2024 Jan 24;27(2):109013. doi: 10.1016/j.isci.2024.109013. eCollection 2024 Feb 16. iScience. 2024. PMID: 38327787 Free PMC article.
-
Ameliorative effects of propolis upon reproductive toxicity in males.Clin Exp Reprod Med. 2023 Mar;50(1):12-18. doi: 10.5653/cerm.2022.05785. Epub 2023 Feb 23. Clin Exp Reprod Med. 2023. PMID: 36935407 Free PMC article.
-
Insight into Potential Interactions of Thyroid Hormones, Sex Hormones and Their Stimulating Hormones in the Development of Non-Alcoholic Fatty Liver Disease.Metabolites. 2022 Aug 4;12(8):718. doi: 10.3390/metabo12080718. Metabolites. 2022. PMID: 36005590 Free PMC article. Review.
-
Sex-Specific Differences in Lipoprotein Production and Clearance.Arterioscler Thromb Vasc Biol. 2023 Sep;43(9):1617-1625. doi: 10.1161/ATVBAHA.122.318247. Epub 2023 Jul 6. Arterioscler Thromb Vasc Biol. 2023. PMID: 37409532 Free PMC article. Review.
References
-
- Persson L., Henriksson P., Westerlund E., Hovatta O., Angelin B., Rudling M. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arteriosclerosis, Thrombosis, and Vascular Biology . 2012;32(3):810–814. doi: 10.1161/atvbaha.111.242461. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

