Progressive Brain Structural Impairment Assessed via Network and Causal Analysis in Patients With Hepatitis B Virus-Related Cirrhosis
- PMID: 35599731
- PMCID: PMC9120530
- DOI: 10.3389/fneur.2022.849571
Progressive Brain Structural Impairment Assessed via Network and Causal Analysis in Patients With Hepatitis B Virus-Related Cirrhosis
Abstract
Objectives: This research amid to elucidate the disease stage-specific spatial patterns and the probable sequences of gray matter (GM) deterioration as well as the causal relationship among structural network components in hepatitis B virus-related cirrhosis (HBV-RC) patients.
Methods: Totally 30 HBV-RC patients and 38 healthy controls (HC) were recruited for this study. High-resolution T1-weighted magnetic resonance imaging and psychometric hepatic encephalopathy score (PHES) were evaluated in all participants. Voxel-based morphometry (VBM), structural covariance network (SCN), and causal SCN (CaSCN) were applied to identify the disease stage-specific GM abnormalities in morphology and network, as well as their causal relationship.
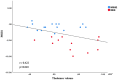
Results: Compared to HC (0.443 ± 0.073 cm3), the thalamus swelled significantly in the no minimal hepatic encephalopathy (NMHE) stage (0.607 ± 0.154 cm3, p <0.05, corrected) and further progressed and expanded to the bilateral basal ganglia, the cortices, and the cerebellum in the MHE stage (p < 0.05, corrected). Furthermore, the thalamus swelling had a causal effect on other parts of cortex-basal ganglia-thalamus circuits (p < 0.05, corrected), which was negatively correlated with cognitive performance (r = -0.422, p < 0.05). Moreover, the thalamus-related SCN also displayed progressive deterioration as the disease advanced in HBV-RC patients (p < 0.05, corrected).
Conclusion: Progressive deterioration of GM morphology and SCN exists in HBV-RC patients during advanced disease, displaying thalamus-related causal effects. These findings indicate that bilateral thalamus morphology as well as the thalamus-related network may serve as an in vivo biomarker for monitoring the progression of the disease in HBV-RC patients.
Keywords: MRI; cirrhosis; gray matter; hepatic encephalopathy; thalamus.
Copyright © 2022 Lin, Guo, Chen, Lin, Ye and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Lu CQ, Jiao Y, Meng XP, Cai Y, Luan Y, Xu XM, et al. Structural change of thalamus in cirrhotic patients with or without minimal hepatic encephalopathy and the relationship between thalamus volume and clinical indexes related to cirrhosis. Neuroimage Clin. (2018) 20:800–7. 10.1016/j.nicl.2018.09.015 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

