Hydrophilic Realgar Nanocrystals Prolong the Survival of Refractory Acute Myeloid Leukemia Mice Through Inducing Multi-Lineage Differentiation and Apoptosis
- PMID: 35599749
- PMCID: PMC9122054
- DOI: 10.2147/IJN.S358469
Hydrophilic Realgar Nanocrystals Prolong the Survival of Refractory Acute Myeloid Leukemia Mice Through Inducing Multi-Lineage Differentiation and Apoptosis
Abstract
Introduction: Acute myeloid leukemia (AML) is a heterogeneous clonal disorder of hematopoietic progenitor cells, and the AML cells are differentiation retarded which results in the hyperproliferation of those malignant tumor cells. To stop the uncontrollable proliferation, inducing the AML cell differentiation is one highly expected therapy because it can bring relatively low systematic side effects compared to conventional chemotherapies; however, there are few options of inductive therapeutics in the clinical applications so far. This study aims to investigate the differentiation-induction effects of lab-developed hydrophilic nanocrystals of As4S4 (ee-As4S4).
Methods: In this work, ee-As4S4 was applied upon a refractory mouse model co-expressing AML1-ETO and HyC-KITD816V as well as a related human AML cell line, Kasumi-1, to investigate whether the nanocrystals can break the retardation of differentiation and drive the cells undergo apoptosis.
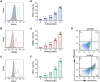
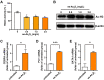
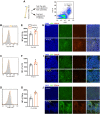
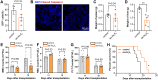
Results: It was shown that ee-As4S4 induced the upregulation of surface markers CD11b, CD235a, and CD41a, which indicate granulocytic, erythroid, and megakaryocytic differentiation respectively, leading to the multiple-lineage differentiation and post-differentiation apoptosis, and the inhibition of histone deacetylase activity was largely involved with the differentiation-induction effects. In the AML mice, orally administered ee-As4S4 increased the level of Ter119, CD11b, and CD41 in bone marrow-derived leukemia cells while reducing the percentage of leukemic cells in the bone marrow. Also, ee-As4S4 improved the hemogram and relieved the hepatomegaly and splenomegaly of the AML mice. As a result, the survival of the AML mice was significantly prolonged. Importantly, ee-As4S4 did not cause acute or chronic toxicity in healthy mice.
Conclusion: In conclusion, ee-As4S4 induced effective and multiple-lineage differentiation and apoptosis of AML cells in the refractory AML mouse model and cell line, suggesting that it holds promising potential as a novel inductive agent in differentiation therapy of AML.
Keywords: As4S4; acute myeloid leukemia; differentiation therapy; histone deacetylation; nanocrystals.
© 2022 Wang et al.
Conflict of interest statement
The authors declare no competing interest in this work.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

