Immuno-Thrombotic Complications of COVID-19: Implications for Timing of Surgery and Anticoagulation
- PMID: 35599794
- PMCID: PMC9119324
- DOI: 10.3389/fsurg.2022.889999
Immuno-Thrombotic Complications of COVID-19: Implications for Timing of Surgery and Anticoagulation
Abstract
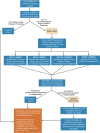
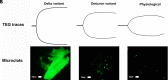
Early in the coronavirus disease 2019 (COVID-19) pandemic, global governing bodies prioritized transmissibility-based precautions and hospital capacity as the foundation for delay of elective procedures. As elective surgical volumes increased, convalescent COVID-19 patients faced increased postoperative morbidity and mortality and clinicians had limited evidence for stratifying individual risk in this population. Clear evidence now demonstrates that those recovering from COVID-19 have increased postoperative morbidity and mortality. These data-in conjunction with the recent American Society of Anesthesiologists guidelines-offer the evidence necessary to expand the early pandemic guidelines and guide the surgeon's preoperative risk assessment. Here, we argue elective surgeries should still be delayed on a personalized basis to maximize postoperative outcomes. We outline a framework for stratifying the individual COVID-19 patient's fitness for surgery based on the symptoms and severity of acute or convalescent COVID-19 illness, coagulopathy assessment, and acuity of the surgical procedure. Although the most common manifestation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is COVID-19 pneumonitis, every system in the body is potentially afflicted by an endotheliitis. This endothelial derangement most often manifests as a hypercoagulable state on admission with associated occult and symptomatic venous and arterial thromboembolisms. The delicate balance between hyper and hypocoagulable states is defined by the local immune-thrombotic crosstalk that results commonly in a hemostatic derangement known as fibrinolytic shutdown. In tandem, the hemostatic derangements that occur during acute COVID-19 infection affect not only the timing of surgical procedures, but also the incidence of postoperative hemostatic complications related to COVID-19-associated coagulopathy (CAC). Traditional methods of thromboprophylaxis and treatment of thromboses after surgery require a tailored approach guided by an understanding of the pathophysiologic underpinnings of the COVID-19 patient. Likewise, a prolonged period of risk for developing hemostatic complications following hospitalization due to COVID-19 has resulted in guidelines from differing societies that recommend varying periods of delay following SARS-CoV-2 infection. In conclusion, we propose the perioperative, personalized assessment of COVID-19 patients' CAC using viscoelastic hemostatic assays and fluorescent microclot analysis.
Keywords: COVID-19; elective surgical procedure; fibrinolysis; immunothrombosis; obstetrics; orthopedic procedures; thrombophilia; venous thromboembolism.
Copyright © 2022 Bunch, Moore, Moore, Neal, Thomas, Zackariya, Zhao, Zackariya, Brenner, Berquist, Buckner, Wiarda, Fulkerson, Huff, Kwaan, Lankowicz, Laubscher, Lourens, Pretorius, Kotze, Moolla, Sithole, Maponga, Kell, Fox, Gillespie, Khan, Mamczak, March, Macias, Bull and Walsh.
Conflict of interest statement
EEM: Research support from Haemonetics, Instrumentation Laboratory, Hemosonics, Stago, and Diapharma. HBM: Research support from Haemonetics and Instumentation Laboratory. MJK is a non-executive director and shareholder of Gknowmix (Pty) Ltd. EP is the managing director of BioCODE Technologies. MMW is on the speaker’s bureau for AstraZeneca.
Figures


References
-
- Mattingly AS, Rose L, Eddington HS, Trickey AW, Cullen MR, Morris AM, et al. Trends in US Surgical Procedures and Health Care System Response to Policies Curtailing Elective Surgical Operations During the COVID-19 Pandemic. JAMA Netw Open. (2021) 4(12):e2138038. 10.1001/jamanetworkopen.2021.38038 - DOI - PMC - PubMed
-
- Collaborative, Irish Surgical Research, Italian Society of Colorectal Surgery, Association of Surgeons in Training, and Transatlantic Australasian Retroperitoneal Sarcoma Working Group. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. (2021) 22(11):1507–17. 10.1016/S1470-2045(21)00493-9 - DOI - PMC - PubMed
-
- American College of Surgeons. COVID-19: recommendations for management of elective surgical procedures. Chicago: ACOS; (2020).
-
- American College of Surgeons. COVID-19: elective case triage guidelines for surgical care. Chicago: ACOS; (2020).
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

