Prevalence of abnormal liver biochemistry and its impact on COVID-19 patients' outcomes: a single-center Greek study
- PMID: 35599935
- PMCID: PMC9062840
- DOI: 10.20524/aog.2022.0709
Prevalence of abnormal liver biochemistry and its impact on COVID-19 patients' outcomes: a single-center Greek study
Abstract
Background: Abnormalities in aminotransferases are frequently observed in hospitalized COVID-19 patients, but their clinical impact is poorly characterized.
Methods: A total of 1046 patients hospitalized to the non-intensive care unit ward with documented COVID-19 were included retrospectively. Demographic, clinical and laboratory characteristics on admission and during hospital stay, including the presence of liver injury (LI), defined as aspartate aminotransferase (AST) >200 IU/L, were recorded.
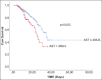
Results: On admission, 363 (34.7%) and 269 (25.7%) patients had abnormal AST and ALT values (i.e., >40 IU/L), respectively, while during hospitalization 53 (5%) patients fulfilled the criteria for LI. In multivariate logistic regression analysis, AST (odds ratio [OR] 1.023, 95% confidence interval [CI] 1.016-1.029; P<0.001), and ferritin (OR 1.01, 95%CI 1.001-1.02; P<0.001) were the baseline factors independently associated with the development of LI during hospital stay. One hundred twenty-three (11.7%) patients died during hospitalization. The independent variables associated with mortality were: age (hazard ratio [HR] 1.043, 95%CI 1.029-1.056; P<0.001), ferritin (HR 1.1, 95%CI 1.05-1.2; P<0.001), platelets (HR 0.996, 95%CI 0.994-0.999; P=0.003), and administration of remdesivir (HR 0.50, 95%CI 0.30-0.85; P=0.009). The patients with abnormal baseline AST (i.e., >40 IU/L), compared to those with normal AST values, had worse outcomes (log rank test: 8.8, P=0.003).
Conclusions: Elevated aminotransferases are commonly seen in COVID-19 patients. They possibly reflect disease severity and may be associated with in-hospital mortality.
Keywords: COVID-19; disease severity; liver function tests; liver injury; mortality.
Copyright: © Hellenic Society of Gastroenterology.
Conflict of interest statement
Conflict of Interest: None
Figures
References
-
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China - key questions for impact assessment. N Engl J Med. 2020;382:692–694. - PubMed
LinkOut - more resources
Full Text Sources