Strategies for Biomaterial-Based Spinal Cord Injury Repair via the TLR4-NF-κB Signaling Pathway
- PMID: 35600111
- PMCID: PMC9116428
- DOI: 10.3389/fbioe.2021.813169
Strategies for Biomaterial-Based Spinal Cord Injury Repair via the TLR4-NF-κB Signaling Pathway
Abstract
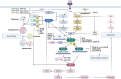
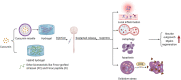
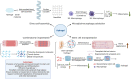
The repair and motor functional recovery after spinal cord injury (SCI) has remained a clinical challenge. Injury-induced gliosis and inflammation lead to a physical barrier and an extremely inhibitory microenvironment, which in turn hinders the recovery of SCI. TLR4-NF-κB is a classic implant-related innate immunomodulation signaling pathway and part of numerous biomaterial-based treatment strategies for SCI. Numerous experimental studies have demonstrated that the regulation of TLR4-NF-κB signaling pathway plays an important role in the alleviation of inflammatory responses, the modulation of autophagy, apoptosis and ferroptosis, and the enhancement of anti-oxidative effect post-SCI. An increasing number of novel biomaterials have been fabricated as scaffolds and carriers, loaded with phytochemicals and drugs, to inhibit the progression of SCI through regulation of TLR4-NF-κB. This review summarizes the empirical strategies for the recovery after SCI through individual or composite biomaterials that mediate the TLR4-NF-κB signaling pathway.
Keywords: TLR4-NF-κB; autophagy; biomaterial; signaling pathway; spinal cord injury.
Copyright © 2022 Lv, Shen, Cheng, Chen, Ding, Yuan, Zhao and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adil M. M., Vazin T., Ananthanarayanan B., Rodrigues G. M. C., Rao A. T., Kulkarni R. U., et al. (2017). Engineered Hydrogels Increase the post-transplantation Survival of Encapsulated hESC-Derived Midbrain Dopaminergic Neurons. Biomaterials 136, 1–11. 10.1016/j.biomaterials.2017.05.008 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

