Sulforaphane Ameliorates the Intestinal Injury in Necrotizing Enterocolitis by Regulating the PI3K/Akt/GSK-3 β Signaling Pathway
- PMID: 35600210
- PMCID: PMC9117068
- DOI: 10.1155/2022/6529842
Sulforaphane Ameliorates the Intestinal Injury in Necrotizing Enterocolitis by Regulating the PI3K/Akt/GSK-3 β Signaling Pathway
Abstract
Objective: Necrotizing enterocolitis (NEC) is a serious neonatal disease; this study aims to investigate the role of sulforaphane (SFN) in NEC-induced intestinal injury.
Methods: An animal model of NEC was established in newborn mice and intragastrically administrated with SFN; then, the general status and survival of the mice were observed. H&E staining was used to observe the pathological changes of intestinal tissues. ELISA, immunohistochemical staining, and flow cytometry assays were used to detect the levels of inflammatory factors, including TNF-α, IL-6, and IL-17, the expression of Bax, Bcl-2, TLR4, and NF-κB, and the percentages of the Th17 and Treg cells, respectively. GSK-3β expression levels were measured by immunofluorescence. IEC-6 and FHC cells were induced with LPS to mimic NEC in vitro and coincubated with SFN; then, the inflammatory factor levels and cell apoptosis rate were detected. Finally, Western blot was used to assess the expression of PI3K/Akt/GSK-3β pathway-related proteins in vitro and in vivo.
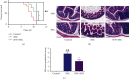
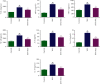
Results: SFN improved the survival rate of NEC mice during modeling, alleviated the severity of the intestinal injury, and reduced the proportion of Th17/Treg cells. SFN could inhibit TLR4 and NF-κB levels, decrease the release of inflammatory factors TNF-α and IL-6, suppress Bax expression, increase Bcl-2 expression, and inhibit apoptosis both in in vitro and in vivo models of NEC. Meanwhile, SFN regulated the expression of PI3K/Akt/GSK-3β pathway-related proteins in vitro and in vivo.
Conclusion: SFN relieved the inflammatory response and apoptosis by regulating the PI3K/Akt/GSK-3β signaling pathway, thereby alleviating NEC in model mice and cells.
Copyright © 2022 Zhong-Kun Bao et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

