Chimeric Antigen Receptor Based Cellular Therapy for Treatment Of T-Cell Malignancies
- PMID: 35600381
- PMCID: PMC9121778
- DOI: 10.3389/fonc.2022.876758
Chimeric Antigen Receptor Based Cellular Therapy for Treatment Of T-Cell Malignancies
Abstract
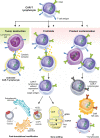
T-cell malignancies can be divided into precursor (T-acute lymphoblastic leukemia/lymphoblastic lymphoma, T-ALL/LBL) and mature T-cell neoplasms, which are comprised of 28 different entities. Most of these malignancies are aggressive with rather poor prognosis. Prognosis of relapsed/refractory (R/R) disease is especially dismal, with an expected survival only several months after progression. Targeted therapies, such as antiCD30 immunotoxin brentuximab vedotin, antiCD38 antibody daratumumab, and anti-CCR4 antibody mogamulizumab are effective only in subsets of patients with T-cell neoplasms. T-cells equipped with chimeric antigen receptor (CAR-Ts) are routinely used for treatment of R/R B-cell malignancies, however, there are specific obstacles for their use in T-cell leukemias and lymphomas which are fratricide killing, risk of transfection of malignant cells, and T-cell aplasia. The solution for these problems relies on target antigen selection, CRISPR/Cas9 or TALEN gene editing, posttranslational regulation of CAR-T surface antigen expression, and safety switches. Structural chromosomal changes and global changes in gene expression were observed with gene-edited products. We identified 49 studies of CAR-based therapies registered on www.clinicaltrials.gov. Most of them target CD30 or CD7 antigen. Results are available only for a minority of these studies. In general, clinical responses are above 50% but reported follow-up is very short. Specific toxicities of CAR-based therapies, namely cytokine release syndrome (CRS), seem to be connected with the antigen of interest and source of cells for manufacturing. CRS is more frequent in antiCD7 CAR-T cells than in antiCD30 cells, but it is mild in most patients. More severe CRS was observed after gene-edited allogeneic CAR-T cells. Immune effector cell associated neurotoxicity (ICANS) was mild and infrequent. Graft-versus-host disease (GvHD) after allogeneic CAR-T cells from previous hematopoietic stem cell donor was also observed. Most frequent toxicities, similarly to antiCD19 CAR-T cells, are cytopenias. CAR-based cellular therapy seems feasible and effective for T-cell malignancies, however, the optimal design of CAR-based products is still unknown and long-term follow-up is needed for evaluation of their true potential.
Keywords: CAR-T cells; T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma; T-cell lymphoma; chimeric antigen receptor (CAR); immunotherapy; therapy.
Copyright © 2022 Polgárová, Otáhal, Šálek and Pytlík.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Gökbuget N, Basara N, Baurmann H, Beck J, Brüggemann M, Diedrich H, et al. High Single-Drug Activity of Nelarabine in Relapsed T-Lymphoblastic Leukemia/Lymphoma Offers Curative Option With Subsequent Stem Cell Transplantation. Blood (2011) 118(13):3504–11. doi: 10.1182/blood-2011-01-329441 - DOI - PubMed
-
- Candoni A, Lazzarotto D, Ferrara F, Curti A, Lussana F, Papayannidis C, et al. Nelarabine as Salvage Therapy and Bridge to Allogeneic Stem Cell Transplant in 118 Adult Patients With Relapsed/Refractory T-Cell Acute Lymphoblastic Leukemia/Lymphoma. A CAMPUS ALL Study. Am J Hematol (2020) 95(12):1466–72. doi: 10.1002/ajh.25957 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

