Liposomes: structure, composition, types, and clinical applications
- PMID: 35600452
- PMCID: PMC9118483
- DOI: 10.1016/j.heliyon.2022.e09394
Liposomes: structure, composition, types, and clinical applications
Abstract
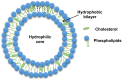
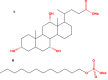
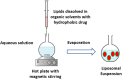
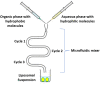
Liposomes are now considered the most commonly used nanocarriers for various potentially active hydrophobic and hydrophilic molecules due to their high biocompatibility, biodegradability, and low immunogenicity. Liposomes also proved to enhance drug solubility and controlled distribution, as well as their capacity for surface modifications for targeted, prolonged, and sustained release. Based on the composition, liposomes can be considered to have evolved from conventional, long-circulating, targeted, and immune-liposomes to stimuli-responsive and actively targeted liposomes. Many liposomal-based drug delivery systems are currently clinically approved to treat several diseases, such as cancer, fungal and viral infections; more liposomes have reached advanced phases in clinical trials. This review describes liposomes structure, composition, preparation methods, and clinical applications.
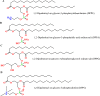
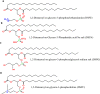
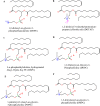
Keywords: Lamellarity; Liposomes; Phospholipids; Stealth liposomes; Vaccinations.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kim B.Y., Rutka J.T., Chan W.C. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2443. - PubMed
-
- Allen T.M., Cullis P.R. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. - PubMed
-
- Khater D., Nsairat H., Odeh F., Saleh M., Jaber A., Alshaer W., Al Bawab A., Mubarak M.S. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: a review. Cosmetics. 2021;8
-
- Yezhelyev M.V., Gao X., Xing Y., Al-Hajj A., Nie S., O'Regan R.M. Emerging use of nanoparticles in diagnosis and treatment of breast cancer, the Lancet. Oncology. 2006;7:657–667. - PubMed
-
- Sahoo S.K., Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today. 2003;8:1112–1120. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

