Finger stick blood test to assess postvaccination SARS-CoV-2 neutralizing antibody response against variants
- PMID: 35600666
- PMCID: PMC9115707
- DOI: 10.1002/btm2.10293
Finger stick blood test to assess postvaccination SARS-CoV-2 neutralizing antibody response against variants
Abstract
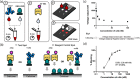
There is clinical need for a quantifiable point-of-care (PoC) SARS-CoV-2 neutralizing antibody (nAb) test that is adaptable with the pandemic's changing landscape. Here, we present a rapid and semi-quantitative nAb test that uses finger stick or venous blood to assess the nAb response of vaccinated population against wild-type (WT), alpha, beta, gamma, and delta variant RBDs. It captures a clinically relevant range of nAb levels, and effectively differentiates prevaccination, post first dose, and post second dose vaccination samples within 10 min. The data observed against alpha, beta, gamma, and delta variants agrees with published results evaluated in established serology tests. Finally, our test revealed a substantial reduction in nAb level for beta, gamma, and delta variants between early BNT162b2 vaccination group (within 3 months) and later vaccination group (post 3 months). This test is highly suited for PoC settings and provides an insightful nAb response in a postvaccinated population.
Keywords: COVID19; SARS‐CoV‐2; cellulose pulldown assay; humoral response against COVID19 variants; neutralizing antibody; point‐of‐care test; serology test.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
Patthara Kongsuphol, Megan E. McBee, Hadley D. Sikes, Huan Jia, and Peter R. Preiser are the co‐founders of Thrixen Pte Ltd, a start‐up company working in further developing some of the technology presented here. Other authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

