A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury
- PMID: 35600969
- PMCID: PMC9108756
- DOI: 10.1016/j.bioactmat.2022.04.029
A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury
Abstract
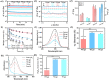
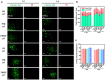
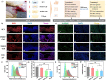
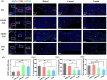
Spinal cord injury (SCI) is an overwhelming and incurable disabling event accompanied by complicated inflammation-related pathological processes, such as excessive reactive oxygen species (ROS) produced by the infiltrated inflammatory immune cells and released to the extracellular microenvironment, leading to the widespread apoptosis of the neuron cells, glial and oligodendroctyes. In this study, a thioketal-containing and ROS-scavenging hydrogel was prepared for encapsulation of the bone marrow derived mesenchymal stem cells (BMSCs), which promoted the neurogenesis and axon regeneration by scavenging the overproduced ROS and re-building a regenerative microenvironment. The hydrogel could effectively encapsulate BMSCs, and played a remarkable neuroprotective role in vivo by reducing the production of endogenous ROS, attenuating ROS-mediated oxidative damage and downregulating the inflammatory cytokines such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), resulting in a reduced cell apoptosis in the spinal cord tissue. The BMSCs-encapsulated ROS-scavenging hydrogel also reduced the scar formation, and improved the neurogenesis of the spinal cord tissue, and thus distinctly enhanced the motor functional recovery of SCI rats. Our work provides a combinational strategy against ROS-mediated oxidative stress, with potential applications not only in SCI, but also in other central nervous system diseases with similar pathological conditions.
Keywords: Anti-oxidation; Axon regeneration; BMSCs; ROS scavenging; Spinal cord injury (SCI).
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Cowan H., Lakra C., Desai M. Autonomic dysreflexia in spinal cord injury. BMJ Br. Med. J. (Clin. Res. Ed.) 2020;371:m3596. - PubMed
-
- Jiang W., Li M., He F., Zhu L. Inhibition of NLRP3 inflammasome attenuates spinal cord injury-induced lung injury in mice. J. Cell. Physiol. 2019;234(5):6012–6022. - PubMed
-
- James S.L., Theadom A., Ellenbogen R.G., Bannick M.S., Montjoy-Venning W., Lucchesi L.R., Abbasi N., Abdulkader R., Abraha H.N., Adsuar J.C., Afarideh M., Agrawal S., et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. - PMC - PubMed
-
- Hutson T.H., Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019;15(12):732–745. - PubMed
-
- Badhiwala J.H., Wilson J.R., Witiw C.D., Harrop J.S., Vaccaro A.R., Aarabi B., Grossman R.G., Geisler F.H., Fehlings M.G. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20(2):117–126. - PubMed
LinkOut - more resources
Full Text Sources

