An immunomodulatory polypeptide hydrogel for osteochondral defect repair
- PMID: 35600970
- PMCID: PMC9112113
- DOI: 10.1016/j.bioactmat.2022.05.008
An immunomodulatory polypeptide hydrogel for osteochondral defect repair
Abstract
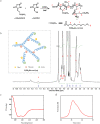
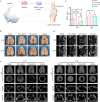
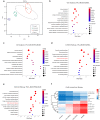
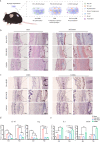
Osteochondral injury is a common and frequent orthopedic disease that can lead to more serious degenerative joint disease. Tissue engineering is a promising modality for osteochondral repair, but the implanted scaffolds are often immunogenic and can induce unwanted foreign body reaction (FBR). Here, we prepare a polypept(o)ide-based PAA-RGD hydrogel using a novel thiol/thioester dual-functionalized hyperbranched polypeptide P(EG3Glu-co-Cys) and maleimide-functionalized polysarcosine under biologically benign conditions. The PAA-RGD hydrogel shows suitable biodegradability, excellent biocompatibility, and low immunogenicity, which together lead to optimal performance for osteochondral repair in New Zealand white rabbits even at the early stage of implantation. Further in vitro and in vivo mechanistic studies corroborate the immunomodulatory role of the PAA-RGD hydrogel, which induces minimum FBR responses and a high level of polarization of macrophages into the immunosuppressive M2 subtypes. These findings demonstrate the promising potential of the PAA-RGD hydrogel for osteochondral regeneration and highlight the importance of immunomodulation. The results may inspire the development of PAA-based materials for not only osteochondral defect repair but also various other tissue engineering and bio-implantation applications.
Keywords: Foreign-body reaction; Immunoregulation; Mesenchymal stem cells; Osteochondral regeneration; Polypeptide hydrogel.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Kunze K.N., Manzi J.S., Wright-Chisem J., Ramkumar P., Nwachukwu B.U., Williams R.R. Risk factors for failure after osteochondral allograft transplantation of the knee: a systematic review and exploratory meta-analysis. Am. J. Sports Med. 2022 doi: 10.1177/03635465211063901. 3635465211063901. - DOI - PubMed
-
- Cao H., Wang X., Chen M., Liu Y., Cui X., Liang J., Wang Q., Fan Y., Zhang X. Childhood cartilage ECM enhances the chondrogenesis of endogenous cells and subchondral bone repair of the unidirectional collagen-dECM scaffolds in combination with microfracture. ACS Appl. Mater. Interfaces. 2021;13:57043–57057. doi: 10.1021/acsami.1c19447. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

