LIN28A alleviates inflammation, oxidative stress, osteogenic differentiation and mineralization in lipopolysaccharide (LPS)-treated human periodontal ligament stem cells
- PMID: 35601075
- PMCID: PMC9117959
- DOI: 10.3892/etm.2022.11338
LIN28A alleviates inflammation, oxidative stress, osteogenic differentiation and mineralization in lipopolysaccharide (LPS)-treated human periodontal ligament stem cells
Abstract
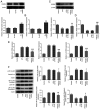
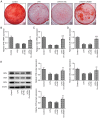
Periodontitis is a complex dental condition that has a number of different etiologies. Lin-28 homolog A (LIN28A) has been previously reported to regulate inflammation, where its expression levels have been indicated to be lower in periodontal tissues following periodontitis. However, there is a lack of evidence to indicate the precise role of LIN28A in periodontitis. In the present study, LIN28A and Runt-related transcription factor 2 (RUNX2) expression were measured in human periodontal biopsy tissues using reverse transcription-quantitative PCR (RT-qPCR). RT-qPCR and western blot analyses were also used to measure LIN28A and RUNX2 expression in human periodontal ligament stem cells (hPDLSCs) following lipopolysaccharide (LPS) induction. Following construction of the LIN28A overexpression plasmid, the expression of LIN28A, RUNX2, osteopontin, osterix and osteocalcin were detected using RT-qPCR and western blotting. Additionally, RT-qPCR was used for the detection of proinflammatory biomarkers (IL-8, IL-1β and IL-6) and alkaline phosphatase (ALP) expression. Protein expression of intranuclear and cytoplasmic NF-κB p65 and NF-κB p65 phosphorylation were assessed using western blot analysis. The expression of antioxidant factors including SOD and GSH were determined using corresponding commercial assay kits. ALP and the mineralization capacity of hPDLSCs were detected by ALP activity assay and Alizarin red staining. The expression of LIN28A was found to be decreased in periodontal biopsy tissues from periodontitis patients compared with normal tissues and LPS-induced hPDLSCs compared with untreated hPDLSCs, which was positively correlated with RUNX2 expression. LIN28A overexpression was revealed to attenuate inflammatory damage and oxidative stress whilst improving ALP active damage, restoring RUNX2 expression and osteoblastic mineralization in LPS-induced hPDLSCs. In conclusion, the present study suggests that LIN28A serves a key role as a mediator of osteoblast differentiation and mineralization. In addition, LIN28A was able to alleviate inflammatory injury and oxidative stress in LPS-induced hPDLSCs.
Keywords: human periodontal ligament stem cells; lin-28 homolog A; lipopolysaccharide; oxidative stress; periodontitis.
Copyright: © Guo et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Effects of sitagliptin activation of the stromal cell-derived factor-1/CXC chemokine receptor 4 signaling pathway on the proliferation, apoptosis, inflammation, and osteogenic differentiation of human periodontal ligament stem cells induced by lipopolysaccharide.Hua Xi Kou Qiang Yi Xue Za Zhi. 2024 Feb 1;42(1):37-45. doi: 10.7518/hxkq.2024.2023213. Hua Xi Kou Qiang Yi Xue Za Zhi. 2024. PMID: 38475949 Free PMC article. Chinese, English.
-
GNAI3 mediated by Lin28A regulates lipopolysaccharide-induced inflammation and osteogenic differentiation in periodontal stem cells by mediating the NF-κB/NLRP3 inflammasome pathway.Arch Oral Biol. 2024 Jul;163:105974. doi: 10.1016/j.archoralbio.2024.105974. Epub 2024 Apr 12. Arch Oral Biol. 2024. PMID: 38636252
-
Effects of nuclear factor-κB signaling pathway on periodontal ligament stem cells under lipopolysaccharide-induced inflammation.Bioengineered. 2022 Mar;13(3):7951-7961. doi: 10.1080/21655979.2022.2051690. Bioengineered. 2022. PMID: 35297308 Free PMC article.
-
PRMT5 inhibition ameliorates inflammation and promotes the osteogenic differentiation of LPS‑induced periodontal stem cells via STAT3/NF‑κB signaling.Exp Ther Med. 2023 Apr 19;25(6):264. doi: 10.3892/etm.2023.11963. eCollection 2023 Jun. Exp Ther Med. 2023. PMID: 37206565 Free PMC article.
-
Gastrodin attenuates lipopolysaccharide-induced inflammation and oxidative stress, and promotes the osteogenic differentiation of human periodontal ligament stem cells through enhancing sirtuin3 expression.Exp Ther Med. 2022 Apr;23(4):296. doi: 10.3892/etm.2022.11225. Epub 2022 Feb 18. Exp Ther Med. 2022. PMID: 35340880 Free PMC article.
Cited by
-
LIN28A attenuates high glucose-induced retinal pigmented epithelium injury through activating SIRT1-dependent autophagy.Int J Ophthalmol. 2023 Sep 18;16(9):1465-1474. doi: 10.18240/ijo.2023.09.13. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37724283 Free PMC article.
-
Effects of sitagliptin activation of the stromal cell-derived factor-1/CXC chemokine receptor 4 signaling pathway on the proliferation, apoptosis, inflammation, and osteogenic differentiation of human periodontal ligament stem cells induced by lipopolysaccharide.Hua Xi Kou Qiang Yi Xue Za Zhi. 2024 Feb 1;42(1):37-45. doi: 10.7518/hxkq.2024.2023213. Hua Xi Kou Qiang Yi Xue Za Zhi. 2024. PMID: 38475949 Free PMC article. Chinese, English.
References
-
- Venugopal P, Koshy T, Lavu V, Ranga Rao S, Ramasamy S, Hariharan S, Venkatesan V. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J Cell Physiol. 2018;233:5877–5884. doi: 10.1002/jcp.26391. - DOI - PubMed
-
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. 2018;45 (Suppl 20):S162–S170. doi: 10.1002/JPER.17-0721. - DOI - PubMed
-
- Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47 (Suppl 22):S4–S60. doi: 10.1111/jcpe.13290. EFP Workshop Participants and Methodological Consultants. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
