Emergence of Neonatal Sepsis Caused by MCR-9- and NDM-1-Co-Producing Enterobacter hormaechei in China
- PMID: 35601097
- PMCID: PMC9120612
- DOI: 10.3389/fcimb.2022.879409
Emergence of Neonatal Sepsis Caused by MCR-9- and NDM-1-Co-Producing Enterobacter hormaechei in China
Abstract
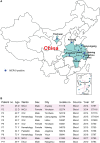
Mobile colistin resistance (mcr) genes represent an emerging threat to public health. Reports on the prevalence, antimicrobial profiles, and clonality of MCR-9-producing Enterobacter cloacae complex (ECC) isolates on a national scale in China are limited. We screened 3,373 samples from humans, animals, and the environment and identified eleven MCR-9-positive ECC isolates. We further investigated their susceptibility, epidemiology, plasmid profiles, genetic features, and virulence potential. Ten strains were isolated from severe bloodstream infection cases, especially three of them were recovered from neonatal sepsis. Enterobacter hormaechei was the most predominant species among the MCR-9-producing ECC population. Moreover, the co-existence of MCR-9, CTX-M, and SHV-12 encoding genes in MCR-9-positive isolates was globally observed. Notably, mcr-9 was mainly carried by IncHI2 plasmids, and we found a novel ~187 kb IncFII plasmid harboring mcr-9, with low similarity with known plasmids. In summary, our study presented genomic insights into genetic characteristics of MCR-9-producing ECC isolates retrieved from human, animal, and environment samples with one health perspective. This study is the first to reveal NDM-1- and MCR-9-co-producing ECC from neonatal sepsis in China. Our data highlights the risk for the hidden spread of the mcr-9 colistin resistance gene.
Keywords: Enterobacter cloacae complex; IncHI2; MCR-9; neonatal; sepsis.
Copyright © 2022 Chen, Xu, Liu, Hu, Han, Wu, Fu, Zheng and Xiao.
Conflict of interest statement
Author JH was employed by Sansure Biotech Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Emergence of concurrently transmissible mcr-9 and carbapenemase genes in bloodborne colistin-resistant Enterobacter cloacae complex isolated from ICU patients in Kolkata, India.Microbiol Spectr. 2025 Mar 4;13(3):e0154224. doi: 10.1128/spectrum.01542-24. Epub 2025 Feb 6. Microbiol Spectr. 2025. PMID: 39912656 Free PMC article.
-
Clonal outbreak of NDM-1-producing Enterobacter hormaechei belonging to high-risk international clone ST78 with the coexistence of tmexCD2-toprJ2 and mcr-9 in China.Int J Antimicrob Agents. 2023 Jun;61(6):106790. doi: 10.1016/j.ijantimicag.2023.106790. Epub 2023 Mar 15. Int J Antimicrob Agents. 2023. PMID: 36924803
-
Molecular characterization of NDM-1-producing carbapenem-resistant E. cloacae complex from a tertiary hospital in Chongqing, China.Front Cell Infect Microbiol. 2022 Aug 8;12:935165. doi: 10.3389/fcimb.2022.935165. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36004335 Free PMC article.
-
Occurrence and Characteristics of Mcrs among Gram-Negative Bacteria Causing Bloodstream Infections of Infant Inpatients between 2006 and 2019 in China.Microbiol Spectr. 2022 Feb 23;10(1):e0193821. doi: 10.1128/spectrum.01938-21. Epub 2022 Feb 9. Microbiol Spectr. 2022. PMID: 35138190 Free PMC article.
-
Detection of Five mcr-9-Carrying Enterobacterales Isolates in Four Czech Hospitals.mSphere. 2020 Dec 9;5(6):e01008-20. doi: 10.1128/mSphere.01008-20. mSphere. 2020. PMID: 33298573 Free PMC article.
Cited by
-
First report of Klebsiella pneumoniae co-producing OXA-181, CTX-M-55, and MCR-8 isolated from the patient with bacteremia.Front Microbiol. 2022 Oct 14;13:1020500. doi: 10.3389/fmicb.2022.1020500. eCollection 2022. Front Microbiol. 2022. PMID: 36312943 Free PMC article.
-
Genomic Analysis of Multidrug Resistant Enterobacter hormaechei Strain AH1-NIMR Isolated from a Neonate with Sepsis in Lagos, Nigeria.Infect Chemother. 2025 Jun;57(2):316-320. doi: 10.3947/ic.2025.0040. Infect Chemother. 2025. PMID: 40618185 Free PMC article.
-
Emergence of an Extensive Drug Resistant Citrobacter portucalensis Clinical Strain Harboring bla SFO-1, bla KPC-2, and bla NDM-1.Infect Drug Resist. 2024 Jun 4;17:2273-2283. doi: 10.2147/IDR.S461118. eCollection 2024. Infect Drug Resist. 2024. PMID: 38854780 Free PMC article.
-
Emergence of concurrently transmissible mcr-9 and carbapenemase genes in bloodborne colistin-resistant Enterobacter cloacae complex isolated from ICU patients in Kolkata, India.Microbiol Spectr. 2025 Mar 4;13(3):e0154224. doi: 10.1128/spectrum.01542-24. Epub 2025 Feb 6. Microbiol Spectr. 2025. PMID: 39912656 Free PMC article.
-
Epidemiology and Risk Factors of Community-Associated Bloodstream Infections in Zhejiang Province, China, 2017-2020.Infect Drug Resist. 2023 Mar 18;16:1579-1590. doi: 10.2147/IDR.S400108. eCollection 2023. Infect Drug Resist. 2023. PMID: 36969944 Free PMC article.
References
-
- Ai W., Zhou Y., Wang B., Zhan Q., Hu L., Xu Y., et al. . (2021). First Report of Coexistence of Bla SFO-1 and Bla NDM-1 Beta-Lactamase Genes as Well as Colistin Resistance Gene Mcr-9 in a Transferrable Plasmid of a Clinical Isolate of Enterobacter Hormaechei. Front. Microbiol. 12, 676113. doi: 10.3389/fmicb.2021.676113 - DOI - PMC - PubMed
-
- Borjesson S., Greko C., Myrenas M., Landen A., Nilsson O., Pedersen K. (2020). A Link Between the Newly Described Colistin Resistance Gene Mcr-9 and Clinical Enterobacteriaceae Isolates Carrying blaSHV-12 From Horses in Sweden. J. Glob. Antimicrob. Resist. 20, 285–289. doi: 10.1016/j.jgar.2019.08.007 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

