Nucleospora hippocampi n. sp., an Intranuclear Microsporidian Infecting the Seahorse Hippocampus erectus From China
- PMID: 35601100
- PMCID: PMC9114889
- DOI: 10.3389/fcimb.2022.882843
Nucleospora hippocampi n. sp., an Intranuclear Microsporidian Infecting the Seahorse Hippocampus erectus From China
Abstract
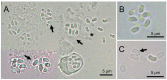
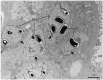
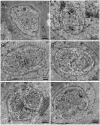
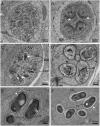
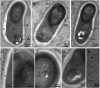
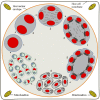
The life cycle, ultrastructure, and molecular phylogeny of a new intranuclear microsporidian, Nucleospora hippocampi n. sp., infecting the intestine of the Hippocampus erectus, were described. The histopathology revealed an extensive infection, mainly in the columnar epithelium of the intestinal mucosa layer. The enterocytes were the important target cell for Nucleospora hippocampi n. sp. infection. Transmission electron microscopy results showed that this microsporidian developed directly within the host cell nucleoplasm. In the intranuclear life cycle, the transformation from meront to sporogonial plasmodium was recognized by forming electron-dense disc structures, which were considered the polar tube precursors. The microsporidian showed the typical morphological characteristics of the family Enterocytozoonidae in the formation and development of spore organelles prior to the division of the sporogonial plasmodium. According to wet smear observation, eight spores were generally formed in a single host nucleus. Mature spores were elongated ovoids that were slightly bent and measured 1.93 × 0.97 μm. The isofilar polar tube was arranged in 7~8 coils in one row. Phylogenetic analysis of its small subunit ribosomal DNA sequences demonstrated that the parasite belonged to the Nucleospora group clade. The histological, ultrastructural, and molecular data support the emergence of a new species in the genus Nucleospora. This is the first report of Nucleospora species in Asia and threatened syngnathid fishes.
Keywords: Nucleospora; intestinal disease; intranuclear parasitism; seahorse; transmission electron microscopy.
Copyright © 2022 Wang, Ying, Huang, Zou, Liu, Li, Zhou, Zhao, Ma, Li, Tan and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Balbiani E. G. (1882). Sur les Microsporidies ou Psorospermies des Articules. Compt. Rend. Acad. Sci. 95, 1168–1171.
-
- Blasiola J. G. (1979). Glugea Heraldi N. Sp. (Microsporida, Glugeidae) From the Seahorse Hippocampus Erectus Perry. J. Fish Dis. 2 (6), 493–500. doi: 10.1111/j.1365-2761.1979.tb00410.x - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

