The Impact of the CTHRSSVVC Peptide Upon Experimental Models of Trypanosoma cruzi Infection
- PMID: 35601101
- PMCID: PMC9121062
- DOI: 10.3389/fcimb.2022.882555
The Impact of the CTHRSSVVC Peptide Upon Experimental Models of Trypanosoma cruzi Infection
Abstract
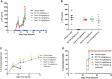
Chagas disease (CD), caused by the hemoflagellate protozoan Trypanosoma cruzi, affects more than six million people worldwide and presents an unsatisfactory therapy, based on two nitroderivatives, introduced in clinical medicine for decades. The synthetic peptide, with CTHRSSVVC sequence (PepA), mimics the CD163 and TNF-α tripeptide "RSS" motif and binds to atheromatous plaques in carotid biopsies of human patients, spleen tissues, and a low-density lipoprotein receptor knockout (LDLr-/-) mouse model of atherosclerosis. CD163 receptor is present on monocytes, macrophages, and neutrophils, acting as a regulator of acute-phase processes and modulating aspects of the inflammatory response and the establishment of infections. Due to the potential theranostic role of PepA, our aim was to investigate its effect upon T. cruzi infection in vitro and in vivo. PepA and two other peptides with shuffled sequences were assayed upon different binomials of host cell/parasite, including professional [as peritoneal mouse macrophages (PMM)] and non-professional phagocytes [primary cultures of cardiac cells (CM)], under different protocols. Also, their impact was further addressed in vivo using a mouse model of acute experimental Chagas disease. Our in-vitro findings demonstrate that PepA and PepB (the peptide with random sequence retaining the "RS" sequence) reduced the intracellular parasitism of the PMM but were inactive during the infection of cardiac cells. Another set of in-vitro and in-vivo studies showed that they do not display a trypanocidal effect on bloodstream trypomastigotes nor exhibit in-vivo efficacy when administered after the parasite inoculation. Our data report the in-vitro activity of PepA and PepB upon the infection of PMM by T. cruzi, possibly triggering the microbicidal arsenal of the host professional phagocytes, capable of controlling parasitic invasion and proliferation.
Keywords: CTHRSSVVC peptide; Chagas disease; Trypanosoma cruzi; experimental chemotherapy; immunomodulation.
Copyright © 2022 Leite, Batista, Mazzeti, Silva, Lugão and Soeiro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Batista D. G., Batista M. M., de Oliveira G. M., do Amaral P. B., Lannes-Vieira J., Britto C. C., et al. . (2010). Arylimidamide DB766, A Potential Chemotherapeutic Candidate for Chagas’ Disease Treatment. Antimicrobial Agents Chemother 54 (7), 2940–2952. doi: 10.1128/AAC.01617-09 - DOI - PMC - PubMed
-
- de Araújo J. S., García-Rubia A., Sebastián-Pérez V., Kalejaiye T. D., da Silva P. B., Fonseca-Berzal C. R., et al. . (2019). Imidazole Derivatives as Promising Agents for the Treatment of Chagas Disease. Antimicrobial Agents Chemother 63 (4), e02156–e02118. doi: 10.1128/AAC.02156-18 - DOI - PMC - PubMed
-
- de Araújo J. S., da Silva C. F., Batista D. G. J., Nefertiti A., de Almeida Fiuza L. F., Fonseca-Berzal C. R., et al. . (2020). Efficacy of Novel Pyrazolone Phosphodiesterase Inhibitors in Experimental Mouse Models of Trypanosoma Cruzi. Antimicrobial Agents Chemother 64 (9), e00414–20. doi: 10.1128/AAC.00414-20 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

