Effect of Helicobacter Pylori Eradication on Human Gastric Microbiota: A Systematic Review and Meta-Analysis
- PMID: 35601105
- PMCID: PMC9114356
- DOI: 10.3389/fcimb.2022.899248
Effect of Helicobacter Pylori Eradication on Human Gastric Microbiota: A Systematic Review and Meta-Analysis
Abstract
Background: Helicobacter pylori (H. pylori) infection is a major risk factor for gastric cancer and eradication of H. pylori is recommended as an effective gastric cancer prevention strategy. The infected individuals show microbial dysbiosis of gastric microbiota. In recent years, agrowing number of studies have focused on gastric microbiota changes following H. pylori eradication. In the present study, we aim to evaluate the influence of successful H. pylori eradication on the short-term and long-term alterations of human gastric microbiota using a method of systematic review and meta-analysis.
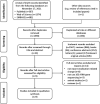
Methods: We did a systematic search based on three databases (PubMed, EMBASE, and Web of Science) in November 2021. Additional articles were also identified by reviewing references cited in the included papers. Human studies that reported changes in gastric microbiota following successful H. pylori eradication were enrolled. PROSPERO registration number: CRD42021293796.
Results: In total, nine studies enrolling 546 participants were included. Regarding quadruple therapy, alpha diversity indexes increased within 1 month after eradication; significant differences in gastric microbial community structure between before and after eradication were also seen within 1 month. The trends of the above-mentioned diversity changes persisted with a follow-up of 6 months. The microbial composition altered significantly after eradication and the relative abundance of H. pylori-related taxa decreased. Accordingly, gastric commonly dominant commensals were enriched. Bioinformatic analyses of microbiota functions showed that bacteria reproduction-related pathways were down-regulated and pathways of gastric acid secretion, etc. were up-regulated. For triple therapy, similar trends of alpha diversity and beta diversity changes were observed in the short-term and long-term follow-up. Also, after eradication, H. pylori was not the gastric dominant bacteria and similar changes in gastric microbial composition were found. For gastric microbial interactions, a decrease in microbial interactions was seen after eradication. Additionally, regarding whether successful H. pylori eradication could restore gastric microbiota to uninfected status, the results remain controversial.
Conclusion: In conclusion, successful H. pylori eradication could reverse the gastric microbiota dysbiosis and show beneficial effects on gastric microbiota. Our findings may provide new insight for exploring the role of H. pylori and the whole gastric microbiota in gastric carcinogenesis.
Keywords: Helicobacter pylori; eradication; gastric microbiota; humans; meta-analysis.
Copyright © 2022 Guo, Cao, Guo, Zhou and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The impact of Helicobacter pylori infection and eradication therapies on gut microbiota: a systematic review of microbial dysbiosis and its implications in gastric carcinogenesis.Front Cell Infect Microbiol. 2025 Jul 7;15:1592977. doi: 10.3389/fcimb.2025.1592977. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40692683 Free PMC article.
-
Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer.Gut. 2020 Sep;69(9):1598-1607. doi: 10.1136/gutjnl-2019-319696. Epub 2019 Dec 19. Gut. 2020. PMID: 31857433 Free PMC article.
-
The eradication of Helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults.Helicobacter. 2019 Aug;24(4):e12590. doi: 10.1111/hel.12590. Epub 2019 May 23. Helicobacter. 2019. PMID: 31124220
-
Gastrointestinal microbiome and Helicobacter pylori: Eradicate, leave it as it is, or take a personalized benefit-risk approach?World J Gastroenterol. 2022 Feb 21;28(7):766-774. doi: 10.3748/wjg.v28.i7.766. World J Gastroenterol. 2022. PMID: 35317277 Free PMC article.
-
Changes in the human gut microbiota composition caused by Helicobacter pylori eradication therapy: A systematic review and meta-analysis.Helicobacter. 2020 Aug;25(4):e12713. doi: 10.1111/hel.12713. Epub 2020 Jun 9. Helicobacter. 2020. PMID: 32515529
Cited by
-
The Role of Probiotics in the Eradication of Helicobacter pylori and Overall Impact on Management of Peptic Ulcer: A Study Involving Patients Undergoing Triple Therapy in Bangladesh.Cureus. 2024 Mar 16;16(3):e56283. doi: 10.7759/cureus.56283. eCollection 2024 Mar. Cureus. 2024. PMID: 38495972 Free PMC article.
-
Simultaneous application of oral and intravaginal probiotics for Helicobacter pylori and its antibiotic-therapy-induced vaginal dysbacteriosis.NPJ Biofilms Microbiomes. 2024 Jun 20;10(1):49. doi: 10.1038/s41522-024-00521-9. NPJ Biofilms Microbiomes. 2024. PMID: 38902244 Free PMC article. Review.
-
Gastric Cancer and Microbiota: Exploring the Microbiome's Role in Carcinogenesis and Treatment Strategies.Life (Basel). 2025 Jun 23;15(7):999. doi: 10.3390/life15070999. Life (Basel). 2025. PMID: 40724502 Free PMC article. Review.
-
Global status and trends of gastric cancer and gastric microbiota research: a bibliometric analysis.Front Microbiol. 2024 Apr 9;15:1341012. doi: 10.3389/fmicb.2024.1341012. eCollection 2024. Front Microbiol. 2024. PMID: 38655079 Free PMC article.
-
Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases.Int J Mol Sci. 2023 Oct 27;24(21):15654. doi: 10.3390/ijms242115654. Int J Mol Sci. 2023. PMID: 37958637 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

