Phenoxazine-Quinoline Conjugates: Impact of Halogenation on Charge Transfer Triplet Energy Harvesting via Aggregate Induced Phosphorescence
- PMID: 35601330
- PMCID: PMC9118413
- DOI: 10.1021/acsomega.2c01909
Phenoxazine-Quinoline Conjugates: Impact of Halogenation on Charge Transfer Triplet Energy Harvesting via Aggregate Induced Phosphorescence
Abstract
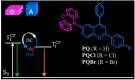
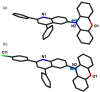
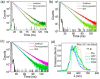
Room-temperature phosphorescence (RTP) from organic compounds has attracted increasing attention in the field of data security, sensing, and bioimaging. However, realization of RTP with an aggregate induced phosphorescence (AIP) feature via harvesting supersensitive excited charge transfer triplet (3CT) energy under visible light excitation (VLE) in single-component organic systems at ambient conditions remains unfulfilled. Organic donor-acceptor (D-A) based orthogonal structures can therefore be used to harvest the energy of the 3CT state at ambient conditions under VLE. Here we report three phenoxazine-quinoline conjugates (PQ, PQCl, PQBr), in which D and A parts are held in orthogonal orientation around the C-N single bond; PQCl and PQBr are substituted with halogens (Cl, Br) while PQ has no halogen atom. Spectroscopic studies and quantum chemistry calculations combining reference compounds (Phx, QPP) reveal that all the compounds in film at ambient conditions show fluorescence and green-RTP due to (i) radiative decay of both singlet charge transfer (1CT) and triplet CT (3CT) states under VLE, (ii) energetic nondegeneracy of 1CT and 3CT states (1CT- 3CT, 0.17-0.21 eV), and (iii) spatial separation of highest and lowest unoccupied molecular orbitals. Further, we found in a tetrahydrofuran-water mixture (f w = 90%, v/v) that both PQCl (10-5 M) and PQBr (10-5 M) show concentration-dependent AIP with phosphorescence quantum yields (ϕP) of ∼25% and ∼28%, respectively, whereas aggregate induced quenching (ACQ) was observed in PQ. The phosphorescence lifetimes (τP) of the PQCl and PQBr aggregates were shown to be ∼22-62 μs and ∼22-59 μs, respectively. The ϕP of the powder samples is found to be 0.03% (PQ), 15.6% (PQCl), and 13.0% (PQBr), which are significantly lower than that of the aggregates (10-5 M, f w = 90%, v/v). Film (Zeonex, 0.1 wt %) studies revealed that ϕP of PQ (7.1%) is relatively high, while PQCl and PQBr exhibit relatively low ϕP values (PQCl, 9.7%; PQBr, 8.8%), as compared with that of powder samples. In addition, we found in single-crystal X-ray analysis that multiple noncovalent interactions along with halogen···halogen (Cl···Cl) interactions between the neighboring molecules play an important role to stabilize the 3CT caused by increased rigidity of the molecular backbone. This design principle reveals a method to understand nondegeneracy of 1CT and 3CT states, and RTP with a concentration-dependent AIP effect using halogen substituted twisted donor-acceptor conjugates.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
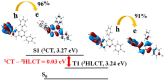


Similar articles
-
Molecular-Level Understanding of Dual-RTP via Host-Sensitized Multiple Triplet-to-Triplet Energy Transfers and Data Security Application.ACS Omega. 2022 Jan 21;7(4):3722-3730. doi: 10.1021/acsomega.1c06390. eCollection 2022 Feb 1. ACS Omega. 2022. PMID: 35128280 Free PMC article.
-
Theory of Long-Lived Room-Temperature Phosphorescence in Organic Aggregates.Acc Chem Res. 2021 Feb 16;54(4):940-949. doi: 10.1021/acs.accounts.0c00556. Epub 2020 Dec 21. Acc Chem Res. 2021. PMID: 33347277
-
Modulation of Delayed Fluorescence Guided by Conformational Effect-Mediated Thermally Enhanced Phosphorescence in Phenothiazines-Quinoline-Cl Conjugates.J Phys Chem B. 2023 Nov 16;127(45):9833-9840. doi: 10.1021/acs.jpcb.3c06274. Epub 2023 Nov 1. J Phys Chem B. 2023. PMID: 37913786
-
Recent Advances in Organic Light-Emitting Diodes Based on Pure Organic Room Temperature Phosphorescence Materials.Front Chem. 2019 May 7;7:305. doi: 10.3389/fchem.2019.00305. eCollection 2019. Front Chem. 2019. PMID: 31134182 Free PMC article. Review.
-
Recent Advances in Impurity-Induced Room-Temperature Phosphorescence.Adv Mater. 2025 Jun 4:e2506549. doi: 10.1002/adma.202506549. Online ahead of print. Adv Mater. 2025. PMID: 40465363 Review.
References
-
- Bhattacharjee I.; Acharya N.; Karmakar S.; Ray D. Room-Temperature Orange-Red Phosphorescence by Way of Intermolecular Charge Transfer in Single-Component Phenoxazine–Quinoline Conjugates and Chemical Sensing. J. Phys. Chem. C 2018, 122, 21589–21597. 10.1021/acs.jpcc.8b06171. - DOI
-
- Baldo M. A.; O’Brien D. F.; You Y.; Shoustikov A.; Sibley S.; Thompson M. E.; Forrest S. R. Highly Efficient Phosphorescent Emission from Organic Electroluminescent Devices. Nature 1998, 395, 151–154. 10.1038/25954. - DOI
-
- Köhler A.; Bässler H. Triplet States in Organic Semiconductors. Mater. Sci. Eng. R Rep. 2009, 66, 71–109. 10.1016/j.mser.2009.09.001. - DOI
-
- Liu Y.; Li C.; Ren Z.; Yan S.; Bryce M. R. All-Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Nat. Rev. Mater. 2018, 3, 18020.10.1038/natrevmats.2018.20. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

