Epithelial-mesenchymal transition: The history, regulatory mechanism, and cancer therapeutic opportunities
- PMID: 35601657
- PMCID: PMC9115588
- DOI: 10.1002/mco2.144
Epithelial-mesenchymal transition: The history, regulatory mechanism, and cancer therapeutic opportunities
Abstract
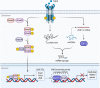
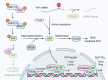
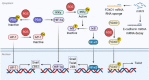
Epithelial-mesenchymal transition (EMT) is a program wherein epithelial cells lose their junctions and polarity while acquiring mesenchymal properties and invasive ability. Originally defined as an embryogenesis event, EMT has been recognized as a crucial process in tumor progression. During EMT, cell-cell junctions and cell-matrix attachments are disrupted, and the cytoskeleton is remodeled to enhance mobility of cells. This transition of phenotype is largely driven by a group of key transcription factors, typically Snail, Twist, and ZEB, through epigenetic repression of epithelial markers, transcriptional activation of matrix metalloproteinases, and reorganization of cytoskeleton. Mechanistically, EMT is orchestrated by multiple pathways, especially those involved in embryogenesis such as TGFβ, Wnt, Hedgehog, and Hippo, suggesting EMT as an intrinsic link between embryonic development and cancer progression. In addition, redox signaling has also emerged as critical EMT modulator. EMT confers cancer cells with increased metastatic potential and drug resistant capacity, which accounts for tumor recurrence in most clinic cases. Thus, targeting EMT can be a therapeutic option providing a chance of cure for cancer patients. Here, we introduce a brief history of EMT and summarize recent advances in understanding EMT mechanisms, as well as highlighting the therapeutic opportunities by targeting EMT in cancer treatment.
Keywords: EMT; cancer progression; embryogenesis; redox signaling; targeted therapy.
© 2022 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Canhua Huang is an editorial board member of MedComm. Author Canhua Huang was not involved in the journal's review of, or decisions related to, this manuscript. The other authors have no conflicts of interest to declare.
Figures





References
-
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial‐mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69‐84. - PubMed
-
- Caramel J, Papadogeorgakis E, Hill L, et al. A switch in the expression of embryonic EMT‐inducers drives the development of malignant melanoma. Cancer Cell. 2013;24(4):466‐480. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
