Setting the Record Straight: A New Twist on the Chemiosmotic Mechanism of Oxidative Phosphorylation
- PMID: 35601666
- PMCID: PMC9112926
- DOI: 10.1093/function/zqac018
Setting the Record Straight: A New Twist on the Chemiosmotic Mechanism of Oxidative Phosphorylation
Figures
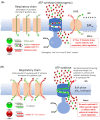
Comment on
-
ATP Synthase K+- and H+-fluxes Drive ATP Synthesis and Enable Mitochondrial K+-"Uniporter" Function: II. Ion and ATP Synthase Flux Regulation.Function (Oxf). 2022 Jan 27;3(2):zqac001. doi: 10.1093/function/zqac001. eCollection 2022. Function (Oxf). 2022. PMID: 35187492 Free PMC article.
-
ATP Synthase K+- and H+-Fluxes Drive ATP Synthesis and Enable Mitochondrial K+-"Uniporter" Function: I. Characterization of Ion Fluxes.Function (Oxf). 2021 Dec 13;3(2):zqab065. doi: 10.1093/function/zqab065. eCollection 2022. Function (Oxf). 2021. PMID: 35229078 Free PMC article.
-
Electrophysiological Experiments Revalidate the Two-ion Theory of Energy Coupling and ATP Synthesis.Function (Oxf). 2022 Feb 14;3(2):zqac004. doi: 10.1093/function/zqac004. eCollection 2022. Function (Oxf). 2022. PMID: 35399498 Free PMC article. No abstract available.
References
-
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191(4784):144–148. - PubMed
-
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966;41(3):445–501. - PubMed
-
- Ferguson SJ. Fully Delocalized Chemiosmotic or Localized Proton Flow Pathways in Energy Coupling - a Scrutiny of Experimental-Evidence. Biochim Biophys Acta. 1985;811(1):47–95.
-
- Ferguson SJ. Chemiosmotic Coupling - Protons Fast and Slow. Curr Biol. 1995;5(1):25–27. - PubMed
-
- Green DE. The electromechanochemical model for energy coupling in mitochondria. Biochim Biophys Acta. 1974;346(1):27–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

