Fusing spheroids to aligned μ-tissues in a heart-on-chip featuring oxygen sensing and electrical pacing capabilities
- PMID: 35601892
- PMCID: PMC9120495
- DOI: 10.1016/j.mtbio.2022.100280
Fusing spheroids to aligned μ-tissues in a heart-on-chip featuring oxygen sensing and electrical pacing capabilities
Abstract
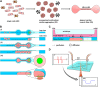
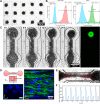
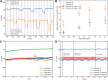
Over the last decade, Organ-on-Chip (OoC) emerged as a promising technology for advanced in vitro models, recapitulating key physiological cues. OoC approaches tailored for cardiac tissue engineering resulted in a variety of platforms, some of which integrate stimulation or probing capabilities. Due to manual handling processes, however, a large-scale standardized and robust tissue generation, applicable in an industrial setting, is still out of reach. Here, we present a novel cell injection and tissue generation concept relying on spheroids, which can be produced in large quantities and uniform size from induced pluripotent stem cell-derived human cardiomyocytes. Hydrostatic flow transports and accumulates spheroids in dogbone-shaped tissue chambers, which subsequently fuse and form aligned, contracting cardiac muscle fibers. Furthermore, we demonstrate electrical stimulation capabilities by utilizing fluidic media connectors as electrodes and provide the blueprint of a low-cost, open-source, scriptable pulse generator. We report on a novel integration strategy of optical O2 sensor spots into resin-based microfluidic systems, enabling in situ determination of O2 partial pressures. Finally, a proof-of-concept demonstrating electrical stimulation combined with in situ monitoring of metabolic activity in cardiac tissues is provided. The developed system thus opens the door for advanced OoCs integrating biophysical stimulation as well as probing capabilities and serves as a blueprint for the facile and robust generation of high density microtissues in microfluidic modules amenable to scaling-up and automation.
Keywords: CMs, Cardiomyocytes; Electrical stimulation; HoC, Heart-on-Chip; Metabolism; Microphysiological systems; Noninvasive readouts; OoC, Organ-on-Chip; Optical sensors; Organ-on-Chip; hiPSCs, human induced pluripotent stem cells.
© 2022 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following competing interest(s): T.M. is a founder, holds equity in PyroScience GmbH in Germany, and is the CEO of the Austrian branch, PyroScience AT GmbH. PyroScience is a developer, producer, and vendor of sensor technology.
Figures



References
-
- Nunes S.S., Miklas J.W., Liu J., Aschar-Sobbi R., Xiao Y., Zhang B., Jiang J., Massé S., Gagliardi M., Hsieh A., Thavandiran N., Laflamme M.A., Nanthakumar K., Gross G.J., Backx P.H., Keller G., Radisic M. Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

