Safety and immunogenicity of Nanocovax, a SARS-CoV-2 recombinant spike protein vaccine: Interim results of a double-blind, randomised controlled phase 1 and 2 trial
- PMID: 35602004
- PMCID: PMC9108376
- DOI: 10.1016/j.lanwpc.2022.100474
Safety and immunogenicity of Nanocovax, a SARS-CoV-2 recombinant spike protein vaccine: Interim results of a double-blind, randomised controlled phase 1 and 2 trial
Abstract
Background: Nanocovax is a recombinant severe acute respiratory syndrome coronavirus 2 subunit vaccine composed of full-length prefusion stabilized recombinant SARS-CoV-2 spike glycoproteins (S-2P) and aluminium hydroxide adjuvant.
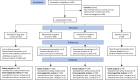
Methods: We conducted a dose-escalation, open label trial (phase 1) and a randomized, double-blind, placebo-controlled trial (phase 2) to evaluate the safety and immunogenicity of the Nanocovax vaccine (in 25 mcg, 50 mcg, and 75 mcg doses, aluminium hydroxide adjuvanted (0·5 mg/dose) in 2-dose regime, 28 days apart (ClinicalTrials.gov number, NCT04683484). In phase 1, 60 participants received two intramuscular injection of the vaccine following dose-escalation procedure. The primary outcomes were reactogenicity and laboratory tests to evaluate the vaccine safety. In phase 2, 560 healthy adults received either vaccine doses similar in phase 1 (25 or 50 or 75 mcg S antigen in 0·5 mg aluminium per dose) or adjuvant (0·5 mg aluminium) in a ratio of 2:2:2:1. One primary outcome was the vaccine safety, including solicited adverse events for 7 day and unsolicited adverse events for 28 days after each injection as well as serious adverse event or adverse events of special interest throughout the study period. Another primary outcome was anti-S IgG antibody response (Index unit/ml). Secondary outcomes were surrogate virus neutralisation (inhibition percentage), wild-type SARS-CoV-2 neutralisation (dilution fold), and T-cell responses by intracellular staining for interferon gamma (IFNg). Anti-S IgG and neutralising antibody levels were compared with convalescent serum samples from symptomatic Covid-19 patients.
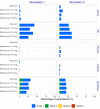
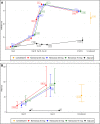
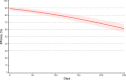
Findings: For phase 1 study, no serious adverse events were observed for all 60 participants. Most adverse events were grade 1 and disappeared shortly after injection. For phase 2 study, after randomisation, 480 participants were assigned to receive the vaccine with adjuvant, and 80 participants were assigned to receive the placebo (adjuvant only). Reactogenicity was absent or mild in the majority of participants and of short duration (mean ≤3 days). Unsolicited adverse events were mild in most participants. There were no serious adverse events related to Nanocovax. Regarding the immunogenicity, Nanocovax induced robust anti-S antibody responses. In general, there humoral responses were similar among vaccine groups which reached their peaks at day 42 and declined afterward. At day 42, IgG levels of vaccine groups were 60·48 [CI95%: 51·12-71·55], 49·11 [41·26-58·46], 57·18 [48·4-67·5] compared to 7·10 [6·32-13·92] of convalescent samples. IgG levels reported here can be converted to WHO international standard binding antibody unit (BAU/ml) by multiplying them to a conversion factor of 21·8. Neutralising antibody titre of vaccine groups at day 42 were 89·2 [52·2-152·3], 80·0 [50·8-125.9] and 95·1 [63·1-143·6], compared to 55·1 [33·4-91·0] of the convalescent group.
Interpretation: Up to day 90, Nanocovax was found to be safe, well tolerated, and induced robust immune responses.
Funding: This work was funded by the Coalition for Epidemic Preparedness Innovations (CEPI), the Ministry of Science and Technology of Vietnam, and Nanogen Pharmaceutical Biotechnology JSC.
Keywords: Immunogenicity; Phase 1 and 2 clinical trial; Protein sub-unit vaccine; SARS-CoV-2; Spike protein.
© 2022 The Author(s).
Conflict of interest statement
TPN, HK, TML, TTNT, TTD, TVV, TTTV, QBPN, VTP, VTT, MTNT, TTTN, PTH, HTH, KDN, CCD, TTU, SMD are employees of Nanogen Pharmaceutical Biotechnology JSC. MTNT, and SMD are authors of a pending patent for Nanocovax.
Figures


Similar articles
-
Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: interim findings from a phase 2, randomised, dose-finding, multicentre study.Lancet Infect Dis. 2022 May;22(5):636-648. doi: 10.1016/S1473-3099(21)00764-7. Epub 2022 Jan 25. Lancet Infect Dis. 2022. PMID: 35090638 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan.Lancet Respir Med. 2021 Dec;9(12):1396-1406. doi: 10.1016/S2213-2600(21)00402-1. Epub 2021 Oct 13. Lancet Respir Med. 2021. PMID: 34655522 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial.Lancet Microbe. 2022 Mar;3(3):e193-e202. doi: 10.1016/S2666-5247(21)00280-9. Epub 2022 Jan 24. Lancet Microbe. 2022. PMID: 35098177 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial.Lancet. 2021 Feb 20;397(10275):682-694. doi: 10.1016/S0140-6736(21)00241-5. Epub 2021 Jan 29. Lancet. 2021. PMID: 33524311 Free PMC article. Clinical Trial.
-
Navigating the Quagmire: Comparison and Interpretation of COVID-19 Vaccine Phase 1/2 Clinical Trials.Vaccines (Basel). 2020 Dec 9;8(4):746. doi: 10.3390/vaccines8040746. Vaccines (Basel). 2020. PMID: 33316990 Free PMC article. Review.
Cited by
-
COVID-19 Vaccines: Where Did We Stand at the End of 2023?Viruses. 2024 Jan 29;16(2):203. doi: 10.3390/v16020203. Viruses. 2024. PMID: 38399979 Free PMC article. Review.
-
The Impact of COVID-19 and Vaccine on the Human Nervous System.Neuroendocrinology. 2022;112(11):1046-1057. doi: 10.1159/000524234. Epub 2022 Mar 22. Neuroendocrinology. 2022. PMID: 35316815 Free PMC article. Review.
-
An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations.Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296211056648. doi: 10.1177/10760296211056648. Clin Appl Thromb Hemost. 2022. PMID: 35167393 Free PMC article. Review.
-
Advanced Vaccine Design Strategies against SARS-CoV-2 and Emerging Variants.Bioengineering (Basel). 2023 Jan 22;10(2):148. doi: 10.3390/bioengineering10020148. Bioengineering (Basel). 2023. PMID: 36829642 Free PMC article. Review.
-
Chemical and biological conjugation strategies for the development of multivalent protein vaccine nanoparticles.Biopolymers. 2023 Aug;114(8):e23563. doi: 10.1002/bip.23563. Epub 2023 Jul 25. Biopolymers. 2023. PMID: 37490564 Free PMC article. Review.
References
-
- Siemens - LD COVID-19-Antibody-Testing-Standardization_Whitepaper v2 152123662.pdf. 2021
-
- COVID live update: 190,075,727 cases and 4,087,371 deaths from the Coronavirus-worldometer [Internet]. [cited 2021 Jul 17]. Available from: https://www.worldometers.info/coronavirus/
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

