Potential Mechanisms of Transmission of Tick-Borne Viruses at the Virus-Tick Interface
- PMID: 35602013
- PMCID: PMC9121816
- DOI: 10.3389/fmicb.2022.846884
Potential Mechanisms of Transmission of Tick-Borne Viruses at the Virus-Tick Interface
Abstract
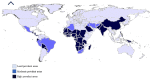
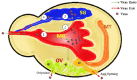
Ticks (Acari; Ixodidae) are the second most important vector for transmission of pathogens to humans, livestock, and wildlife. Ticks as vectors for viruses have been reported many times over the last 100 years. Tick-borne viruses (TBVs) belong to two orders (Bunyavirales and Mononegavirales) containing nine families (Bunyaviridae, Rhabdoviridae, Asfarviridae, Orthomyxovirida, Reoviridae, Flaviviridae, Phenuviridae, Nyamiviridae, and Nairoviridae). Among these TBVs, some are very pathogenic, causing huge mortality, and hence, deserve to be covered under the umbrella of one health. About 38 viral species are being transmitted by <10% of the tick species of the families Ixodidae and Argasidae. All TBVs are RNA viruses except for the African swine fever virus from the family Asfarviridae. Tick-borne viral diseases have also been classified as an emerging threat to public health and animals, especially in resource-poor communities of the developing world. Tick-host interaction plays an important role in the successful transmission of pathogens. The ticks' salivary glands are the main cellular machinery involved in the uptake, settlement, and multiplication of viruses, which are required for successful transmission into the final host. Furthermore, tick saliva also participates as an augmenting tool during the physiological process of transmission. Tick saliva is an important key element in the successful transmission of pathogens and contains different antimicrobial proteins, e.g., defensin, serine, proteases, and cement protein, which are key players in tick-virus interaction. While tick-virus interaction is a crucial factor in the propagation of tick-borne viral diseases, other factors (physiological, immunological, and gut flora) are also involved. Some immunological factors, e.g., toll-like receptors, scavenger receptors, Janus-kinase (JAK-STAT) pathway, and immunodeficiency (IMD) pathway are involved in tick-virus interaction by helping in virus assembly and acting to increase transmission. Ticks also harbor some endogenous viruses as internal microbial faunas, which also play a significant role in tick-virus interaction. Studies focusing on tick saliva and its role in pathogen transmission, tick feeding, and control of ticks using functional genomics all point toward solutions to this emerging threat. Information regarding tick-virus interaction is somewhat lacking; however, this information is necessary for a complete understanding of transmission TBVs and their persistence in nature. This review encompasses insight into the ecology and vectorial capacity of tick vectors, as well as our current understanding of the predisposing, enabling, precipitating, and reinforcing factors that influence TBV epidemics. The review explores the cellular, biochemical, and immunological tools which ensure and augment successful evading of the ticks' defense systems and transmission of the viruses to the final hosts at the virus-vector interface. The role of functional genomics, proteomics, and metabolomics in profiling tick-virus interaction is also discussed. This review is an initial attempt to comprehensively elaborate on the epidemiological determinants of TBVs with a focus on intra-vector physiological processes involved in the successful execution of the docking, uptake, settlement, replication, and transmission processes of arboviruses. This adds valuable data to the existing bank of knowledge for global stakeholders, policymakers, and the scientific community working to devise appropriate strategies to control ticks and TBVs.
Keywords: immunity; salivary glands; tick microbes; tick-virus interaction; ticks.
Copyright © 2022 Maqbool, Sajid, Saqib, Anjum, Tayyab, Rizwan, Rashid, Rashid, Iqbal, Siddique, Shamim, Hassan, Atif, Razzaq, Zeeshan, Hussain, Nisar, Tanveer, Younas, Kamran and Rahman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The antiviral immunity of ticks against transmitted viral pathogens.Dev Comp Immunol. 2021 Jun;119:104012. doi: 10.1016/j.dci.2021.104012. Epub 2021 Jan 21. Dev Comp Immunol. 2021. PMID: 33484780 Review.
-
Tick-borne viruses.Parasitology. 2004;129 Suppl:S221-45. doi: 10.1017/s0031182004005220. Parasitology. 2004. PMID: 15938513 Review.
-
The Known and Unknown of Global Tick-Borne Viruses.Viruses. 2024 Nov 21;16(12):1807. doi: 10.3390/v16121807. Viruses. 2024. PMID: 39772118 Free PMC article. Review.
-
Tick-borne viruses.Acta Virol. 2017;61(4):413-427. doi: 10.4149/av_2017_403. Acta Virol. 2017. PMID: 29186958 Review.
-
Tick-Borne Viruses and Biological Processes at the Tick-Host-Virus Interface.Front Cell Infect Microbiol. 2017 Jul 26;7:339. doi: 10.3389/fcimb.2017.00339. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28798904 Free PMC article. Review.
Cited by
-
Viral Diversity and Ecological Impact of DNA Viruses in Dominant Tick Species in China.Microorganisms. 2024 Aug 22;12(8):1736. doi: 10.3390/microorganisms12081736. Microorganisms. 2024. PMID: 39203578 Free PMC article.
-
The silent spread: uncovering the diversity and evolution of poxviruses in ticks across Western China's host landscapes.Virol J. 2025 Jun 30;22(1):214. doi: 10.1186/s12985-025-02844-1. Virol J. 2025. PMID: 40588758 Free PMC article.
-
Discovery of the Role of Tick Salivary Glands in Enhancement of Virus Transmission-Beginning of an Exciting Story.Pathogens. 2023 Feb 16;12(2):334. doi: 10.3390/pathogens12020334. Pathogens. 2023. PMID: 36839606 Free PMC article.
-
Distribution pattern of Crimean-Congo Hemorrhagic Fever in Asia and the Middle East.Front Public Health. 2023 Jan 26;11:1093817. doi: 10.3389/fpubh.2023.1093817. eCollection 2023. Front Public Health. 2023. PMID: 36778537 Free PMC article. Review.
-
Strain-Dependent Assessment of Powassan Virus Transmission to Ixodes scapularis Ticks.Viruses. 2024 May 23;16(6):830. doi: 10.3390/v16060830. Viruses. 2024. PMID: 38932123 Free PMC article.
References
-
- Ali A., Khan S., Ali I., Karim S., da Silva Vaz I., Termignoni C. (2015b). Probing the functional role of tick metalloproteases. Physiol. Entomol. 40, 177–188. 10.1111/phen.12104 - DOI
-
- Assumpção T. C., Mizurini D. M., Ma D., Monteiro R. Q., Ahlstedt S., Reyes M., et al. . (2018). Ixonnexin from tick saliva promotes fibrinolysis by interacting with plasminogen and tissue-type plasminogen activator, and prevents arterial thrombosis. Sci. Rep. 8, 4806. 10.1038/s41598-018-22780-1 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

