Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica
- PMID: 35602020
- PMCID: PMC9117966
- DOI: 10.3389/fmicb.2022.885092
Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica
Abstract
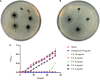
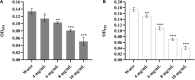
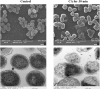
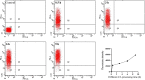
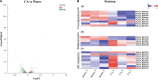
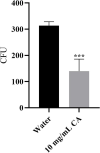
Nowadays, developing new and natural compounds with antibacterial activities from plants has become a promising approach to solve antibiotic resistance of pathogenic bacteria. Chlorogenic acid (CA), as a kind of phenolic acid existing in many plants, has been found to process multifunctional activities including antibacterial activity. Herein, the antibacterial and antibiofilm activities of CA against Yersinia enterocolitica (Y. enterocolitica) were tested for the first time, and its mechanism of action was investigated. It was demonstrated that CA could exert outstanding antibacterial activity against Y. enterocolitica. Biofilm susceptibility assays further indicated that CA could inhibit biofilm formation and decrease the established biofilm biomass of Y. enterocolitica. It was deduced that through binding to Y. enterocolitica, CA destroyed the cell membrane, increased the membrane permeability, and led to bacterial cell damage. In addition, the transcriptomic analysis revealed that CA could disorder many physiological pathways, mainly including the ones of antagonizing biofilms and increasing cell membrane permeability. Finally, the spiked assay showed that the growth of Y. enterocolitica in milk was significantly inhibited by CA. Taken together, CA, as an effective bactericidal effector with application potential, exerts antagonistic activity against Y. enterocolitica by mainly intervening biofilm formation and membrane permeability-related physiological pathways.
Keywords: Yersinia enterocolitica; antibacterial activity; antibiofilm activity; antibiotic resistance; chlorogenic acid.
Copyright © 2022 Chen, Peng, Chi, Yu, Yang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Evaluation of the Membrane Damage Mechanism of Chlorogenic Acid against Yersinia enterocolitica and Enterobacter sakazakii and Its Application in the Preservation of Raw Pork and Skim Milk.Molecules. 2021 Nov 8;26(21):6748. doi: 10.3390/molecules26216748. Molecules. 2021. PMID: 34771154 Free PMC article.
-
Yersinia enterocolitica-Derived Outer Membrane Vesicles Inhibit Initial Stage of Biofilm Formation.Microorganisms. 2022 Nov 29;10(12):2357. doi: 10.3390/microorganisms10122357. Microorganisms. 2022. PMID: 36557609 Free PMC article.
-
The role of osmoregulated periplasmic glucans in the biofilm antibiotic resistance of Yersinia enterocolitica.Microb Pathog. 2020 Oct;147:104284. doi: 10.1016/j.micpath.2020.104284. Epub 2020 May 31. Microb Pathog. 2020. PMID: 32492459
-
Yersinia enterocolitica, a primary model for bacterial invasiveness.Rev Infect Dis. 1987 Jan-Feb;9(1):64-87. doi: 10.1093/clinids/9.1.64. Rev Infect Dis. 1987. PMID: 3547579 Review.
-
Yersinia enterocolitica in milk and dairy products.J Dairy Sci. 1987 Feb;70(2):383-91. doi: 10.3168/jds.S0022-0302(87)80021-8. J Dairy Sci. 1987. PMID: 3553254 Review.
Cited by
-
Staphylococcus aureus biofilm: Formulation, regulatory, and emerging natural products-derived therapeutics.Biofilm. 2024 Jan 1;7:100175. doi: 10.1016/j.bioflm.2023.100175. eCollection 2024 Jun. Biofilm. 2024. PMID: 38298832 Free PMC article. Review.
-
Biological Activities of Galanthus fosteri Extracts: First Demonstration of the Interaction between Chlorogenic Acid and DNA Ligase by Molecular Docking.ACS Omega. 2024 Feb 23;9(10):12254-12261. doi: 10.1021/acsomega.4c00162. eCollection 2024 Mar 12. ACS Omega. 2024. PMID: 38496935 Free PMC article.
-
Utilization of the Shensheng-Piwen changed medicinal powder extracts combines metal-organic frameworks as an antibacterial agent.Front Cell Infect Microbiol. 2024 Jun 7;14:1376312. doi: 10.3389/fcimb.2024.1376312. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38912207 Free PMC article.
-
Isochlorogenic Acid Glucosides from the Arabian Medicinal Plant Artemisia sieberi and Their Antimicrobial Activities.Molecules. 2023 Nov 7;28(22):7460. doi: 10.3390/molecules28227460. Molecules. 2023. PMID: 38005182 Free PMC article.
-
Inula salicina L.: Insights into Its Polyphenolic Constituents and Biological Activity.Pharmaceuticals (Basel). 2024 Jun 27;17(7):844. doi: 10.3390/ph17070844. Pharmaceuticals (Basel). 2024. PMID: 39065695 Free PMC article.
References
-
- Alsolmei F. A., Li H., Pereira S. L., Krishnan P., Johns P. W., Siddiqui R. A. (2019). Polyphenol-enriched plum extract enhances myotubule formation and anabolism while attenuating colon cancer-induced cellular damage in C2C12 cells. Nutrients 11:1077. Epub 2019/05/18. PubMed ; PubMed Central PMCID: PMCPMC6566394 10.3390/nu11051077 - DOI - PMC - PubMed
-
- Bottone E. J. (2015). Yersinia enterocolitica: revisitation of an enduring human pathogen. Clin. Microbiol. Newslett. 37 1–8. 10.1016/j.clinmicnews.2014.12.003 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

