Double-Stranded RNA Viruses Are Released From Trichomonas vaginalis Inside Small Extracellular Vesicles and Modulate the Exosomal Cargo
- PMID: 35602021
- PMCID: PMC9114709
- DOI: 10.3389/fmicb.2022.893692
Double-Stranded RNA Viruses Are Released From Trichomonas vaginalis Inside Small Extracellular Vesicles and Modulate the Exosomal Cargo
Abstract
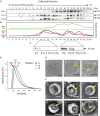
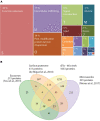
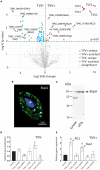
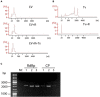
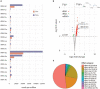
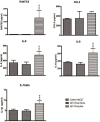
Trichomonas vaginalis is a parasitic protist that infects the human urogenital tract. During the infection, trichomonads adhere to the host mucosa, acquire nutrients from the vaginal/prostate environment, and release small extracellular vesicles (sEVs) that contribute to the trichomonad adherence and modulate the host-parasite communication. Approximately 40-70% of T. vaginalis strains harbor a double-stranded RNA virus called Trichomonasvirus (TVV). Naked TVV particles have the potential to stimulate a proinflammatory response in human cells, however, the mode of TVV release from trichomonads to the environment is not clear. In this report, we showed for the first time that TVV particles are released from T. vaginalis cells within sEVs. The sEVs loaded with TVV stimulated a higher proinflammatory response of human HaCaT cells in comparison to sEVs from TVV negative parasites. Moreover, a comparison of T. vaginalis isogenic TVV plus and TVV minus clones revealed a significant impact of TVV infection on the sEV proteome and RNA cargo. Small EVs from TVV positive trichomonads contained 12 enriched and 8 unique proteins including membrane-associated BspA adhesine, and about a 2.5-fold increase in the content of small regulatory tsRNA. As T. vaginalis isolates are frequently infected with TVV, the release of TVV via sEVs to the environment represents an important factor with the potential to enhance inflammation-related pathogenesis during trichomoniasis.
Keywords: TVV; Trichomonasvirus; exosome; extracellular vesicle; proteomics; tsRNA.
Copyright © 2022 Rada, Hrdý, Zdrha, Narayanasamy, Smutná, Horáčková, Harant, Beneš, Ong, Tsai, Luo, Chiu, Tang and Tachezy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Artuyants A., Campos T. L., Rai A. K., Johnson P. J., Dauros-Singorenko P., Phillips A., et al. (2020). Extracellular vesicles produced by the protozoan parasite Trichomonas vaginalis contain a preferential cargo of tRNA-derived small RNAs. Int. J. Parasitol. 50 1145–1155. 10.1016/J.IJPARA.2020.07.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

