Heavy Metal-Resistant Plant Growth-Promoting Citrobacter werkmanii Strain WWN1 and Enterobacter cloacae Strain JWM6 Enhance Wheat (Triticum aestivum L.) Growth by Modulating Physiological Attributes and Some Key Antioxidants Under Multi-Metal Stress
- PMID: 35602039
- PMCID: PMC9120770
- DOI: 10.3389/fmicb.2022.815704
Heavy Metal-Resistant Plant Growth-Promoting Citrobacter werkmanii Strain WWN1 and Enterobacter cloacae Strain JWM6 Enhance Wheat (Triticum aestivum L.) Growth by Modulating Physiological Attributes and Some Key Antioxidants Under Multi-Metal Stress
Abstract
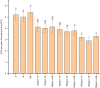
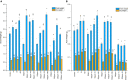
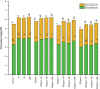
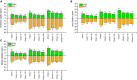
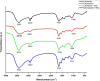
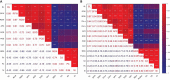
Due to wastewater irrigation, heavy metal (HM) exposure of agricultural soils is a major limiting factor for crop productivity. Plant growth-promoting bacteria (PGPB) may lower the risk of HM toxicity and increase crop yield. In this context, we evaluated two HM-resistant PGPB strains, i.e., Citrobacter werkmanii strain WWN1 and Enterobacter cloacae strain JWM6 isolated from wastewater-irrigated agricultural soils, for their efficacy to mitigate HM (Cd, Ni, and Pb) stress in a pot experiment. Increasing concentrations (0, 50, 100, and 200 ppm) of each HM were used to challenge wheat plants. Heavy metal stress negatively affected wheat growth, biomass, and physiology. The plants under elevated HM concentration accumulated significantly higher amounts of heavy metals (HMs) in shoots and roots, resulting in increased oxidative stress, which was evident from increased malondialdehyde (MDA) content in roots and shoots. Moreover, alterations in antioxidants like superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT) were observed in plants under HM stress. The severity of damage was more pronounced with rising HM concentration. However, inoculating wheat with Citrobacter werkmanii strain WWN1 and Enterobacter cloacae strain JWM6 (107 CFU ml-1) improved plant shoot length (11-42%), root length (19-125%), fresh weight (41-143%), dry weight (65-179%), and chlorophyll a (14%-24%) and chlorophyll b content (2-24%) under HM stress. Citrobacter werkmanii strain WWN1 and Enterobacter cloacae strain JWM6 either alone or in co-inoculation enhanced the antioxidant enzyme activity, which may lower oxidative stress in plants. However, seeds treated with the bacterial consortium showed an overall better outcome in altering oxidative stress and decreasing HM accumulation in wheat shoot and root tissues. Fourier transform infrared spectroscopy indicated the changes induced by HMs in functional groups on the biomass surface that display effective removal of HMs from aqueous medium using PGPB. Thus, the studied bacterial strains may have adequate fertilization and remediation potential for wheat cultivated in wastewater-irrigated soils. However, molecular investigation of mechanisms adopted by these bacteria to alleviate HM stress in wheat is required to be conducted.
Keywords: Citrobacter werkmanii and Enterobacter cloacae; bioremediation and biofertilization; cadmium (Cd); heavy metals contamination; lead (Pb); nickle (Ni); plant growth promoting bacteria; wastewater irrigated agricultural soils.
Copyright © 2022 Ajmal, Yasmin, Hassan, Khan, Jan and Mumtaz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ahanger M. A., Aziz U., Alsahli A. A., Alyemeni M. N., Ahmad P. (2020). Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules 10:147. 10.3390/biom10010147 - DOI - PMC - PubMed
-
- Ahmad H. T., Hussain A., Aimen A., Jamshaid M. U., Ditta A., Asghar H. N., et al. (2021). “Improving resilience against drought stress among crop plants through inoculation of plant growth-promoting rhizobacteria,” in Harsh Environment and Plant Resilience: Molecular and Functional Aspects, 1st Edn, eds Husen A., Jawaid M. (Berlin: Springer; ), 387–408. 10.1007/978-3-030-65912-7_16 - DOI
-
- Ahmad P., Tripathi D. K., Deshmukh R., Singh V. P., Corpas F. J. (2019). Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot. 161 1–3. 10.1016/j.envexpbot.2019.02.017 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

