Specific ablation of PDGFRβ-overexpressing pericytes with antibody-drug conjugate potently inhibits pathologic ocular neovascularization in mouse models
- PMID: 35602228
- PMCID: PMC9053257
- DOI: 10.1038/s43856-021-00059-3
Specific ablation of PDGFRβ-overexpressing pericytes with antibody-drug conjugate potently inhibits pathologic ocular neovascularization in mouse models
Abstract
Background: Crosstalk between pericytes and endothelial cells is critical for ocular neovascularization. Endothelial cells secrete platelet-derived growth factor (PDGF)-BB and recruit PDGF receptor β (PDGFRβ)-overexpressing pericytes, which in turn cover and stabilize neovessels, independent of vascular endothelial growth factor (VEGF). Therapeutic agents inhibiting PDGF-BB/PDGFRβ signaling were tested in clinical trials but failed to provide additional benefits over anti-VEGF agents. We tested whether an antibody-drug conjugate (ADC) - an engineered monoclonal antibody linked to a cytotoxic agent - could selectively ablate pericytes and suppress retinal and choroidal neovascularization.
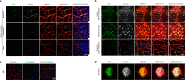
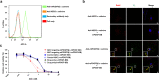
Methods: Immunoblotting, flow cytometry, cell viability test, and confocal microscopy were conducted to assess the internalization and cytotoxic effect of ADC targeting mPDGFRβ in an in vitro setting. Immunofluorescence staining of whole-mount retinas and retinal pigment epithelium-choroid-scleral complexes, electroretinography, and OptoMotry test were used to evaluate the effect and safety of ADC targeting mPDGFRβ in the mouse models of pathologic ocular neovascularization.
Results: ADC targeting mPDGFRβ is effectively internalized into mouse brain vascular pericytes and showed significant cytotoxicity compared with the control ADC. We also show that specific ablation of PDGFRβ-overexpressing pericytes using an ADC potently inhibits pathologic ocular neovascularization in mouse models of oxygen-induced retinopathy and laser-induced choroidal neovascularization, while not provoking generalized retinal toxicity.
Conclusion: Our results suggest that removing PDGFRβ-expressing pericytes by an ADC targeting PDGFRβ could be a potential therapeutic strategy for pathologic ocular neovascularization.
Keywords: Drug delivery; Target validation.
© The Author(s) 2021.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

