A case report describing the immune response of an infant with congenital heart disease and severe COVID-19
- PMID: 35602234
- PMCID: PMC9053208
- DOI: 10.1038/s43856-021-00047-7
A case report describing the immune response of an infant with congenital heart disease and severe COVID-19
Abstract
Background: Children with SARS-CoV-2 infection generally present with milder symptoms or are asymptomatic in comparison with adults, however severe disease occurs in a subset of children. To date, the immune correlates of severe COVID-19 in young children have been poorly characterised.
Methods: We report the kinetics of immune responses in relation to clinical and virological features in an infant with acute severe COVID-19 using high-dimensional flow cytometry and multiplex cytokine analysis.
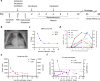
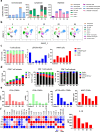
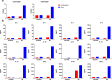
Results: Systemic cellular and cytokine profiling show an initial increase in neutrophils and monocytes with depletion of lymphoid cell populations (particularly CD8 + T and NK cells) and elevated inflammatory cytokines. Expansion of memory CD4 + T (but not CD8 + T) cells occurred over time, with a predominant Th2 bias. Marked activation of T cell populations observed during the acute infection gradually resolved as the child recovered. Substantial in vitro activation of T-cell populations and robust cytokine production, in response to inactivated SARS-CoV-2 stimulation, was observed 3 months after infection indicating durable, long-lived cellular immune memory.
Conclusions: These findings provide important insights into the immune response of a young infant with severe COVID-19 and will help to inform future research into therapeutic targets for high-risk groups.
Keywords: Paediatric research; Viral infection.
© The Author(s) 2021.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

