Rapid Generation of In-House Serological Assays Is Comparable to Commercial Kits Critical for Early Response to Pandemics: A Case With SARS-CoV-2
- PMID: 35602487
- PMCID: PMC9121123
- DOI: 10.3389/fmed.2022.864972
Rapid Generation of In-House Serological Assays Is Comparable to Commercial Kits Critical for Early Response to Pandemics: A Case With SARS-CoV-2
Abstract
Introduction: Accurate and sensitive measurement of antibodies is critical to assess the prevalence of infection, especially asymptomatic infection, and to analyze the immune response to vaccination during outbreaks and pandemics. A broad variety of commercial and in-house serological assays are available to cater to different laboratory requirements; however direct comparison is necessary to understand utility.
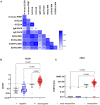
Materials and methods: We investigate the performance of six serological methods against SARS-CoV-2 to determine the antibody profile of 250 serum samples, including 234 RT-PCR-confirmed SARS-CoV-2 cases, the majority with asymptomatic presentation (87.2%) at 1-51 days post laboratory diagnosis. First, we compare to the performance of two in-house antibody assays: (i) an in-house IgG ELISA, utilizing UV-inactivated virus, and (ii) a live-virus neutralization assay (PRNT) using the same Cambodian isolate as the ELISA. In-house assays are then compared to standardized commercial anti-SARS-CoV-2 electrochemiluminescence immunoassays (Elecsys ECLIAs, Roche Diagnostics; targeting anti-N and anti-S antibodies) along with a flow cytometry based assay (FACS) that measures IgM and IgG against spike (S) protein and a multiplex microsphere-based immunoassay (MIA) determining the antibodies against various spike and nucleoprotein (N) antigens of SARS-CoV-2 and other coronaviruses (SARS-CoV-1, MERS-CoV, hCoVs 229E, NL63, HKU1).
Results: Overall, specificity of assays was 100%, except for the anti-S IgM flow cytometry based assay (96.2%), and the in-house IgG ELISA (94.2%). Sensitivity ranged from 97.3% for the anti-S ECLIA down to 76.3% for the anti-S IgG flow cytometry based assay. PRNT and in-house IgG ELISA performed similarly well when compared to the commercial ECLIA: sensitivity of ELISA and PRNT was 94.7 and 91.1%, respectively, compared to S- and N-targeting ECLIA with 97.3 and 96.8%, respectively. The MIA revealed cross-reactivity of antibodies from SARS-CoV-2-infected patients to the nucleocapsid of SARS-CoV-1, and the spike S1 domain of HKU1.
Conclusion: In-house serological assays, especially ELISA and PRNT, perform similarly to commercial assays, a critical factor in pandemic response. Selection of suitable immunoassays should be made based on available resources and diagnostic needs.
Keywords: ELISA; PRNT; SARS-CoV-2; immunoassay; serology.
Copyright © 2022 Auerswald, Eng, Lay, In, Eng, Vo, Sith, Cheng, Delvallez, Mich, Meng, Sovann, Sidonn, Vanhomwegen, Cantaert, Dussart, Duong and Karlsson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- U.S. Food and Drug Administration . EUA Authorized Serology Test Performance. (2021). FDA. Available online at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em... (accessed September 28, 2021).
LinkOut - more resources
Full Text Sources
Miscellaneous

