Age Dependent Changes in Corneal Epithelial Cell Signaling
- PMID: 35602595
- PMCID: PMC9117764
- DOI: 10.3389/fcell.2022.886721
Age Dependent Changes in Corneal Epithelial Cell Signaling
Abstract
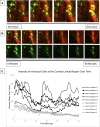
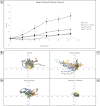
The cornea is exposed daily to a number of mechanical stresses including shear stress from tear film and blinking. Over time, these stressors can lead to changes in the extracellular matrix that alter corneal stiffness, cell-substrate structures, and the integrity of cell-cell junctions. We hypothesized that changes in tissue stiffness of the cornea with age may alter calcium signaling between cells after injury, and the downstream effects of this signaling on cellular motility and wound healing. Nanoindentation studies revealed that there were significant differences in the stiffness of the corneal epithelium and stroma between corneas of 9- and 27-week mice. These changes corresponded to differences in the timeline of wound healing and in cell signaling. Corneas from 9-week mice were fully healed within 24 h. However, the wounds on corneas from 27-week mice remained incompletely healed. Furthermore, in the 27-week cohort there was no detectable calcium signaling at the wound in either apical or basal corneal epithelial cells. This is in contrast to the young cohort, where there was elevated basal cell activity relative to background levels. Cell culture experiments were performed to assess the roles of P2Y2, P2X7, and pannexin-1 in cellular motility during wound healing. Inhibition of P2Y2, P2X7, or pannexin-1 all significantly reduce wound closure. However, the inhibitors all have different effects on the trajectories of individual migrating cells. Together, these findings suggest that there are several significant differences in the stiffness and signaling that underlie the decreased wound healing efficacy of the cornea in older mice.
Keywords: calcium mobilization; cell-cell communication; cornea; live cell imaging; stiffness.
Copyright © 2022 Segars, Azzari, Gomez, Machen, Rich and Trinkaus-Randall.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

