Differentiating amyloid beta spread in autosomal dominant and sporadic Alzheimer's disease
- PMID: 35602652
- PMCID: PMC9116976
- DOI: 10.1093/braincomms/fcac085
Differentiating amyloid beta spread in autosomal dominant and sporadic Alzheimer's disease
Abstract
Amyloid-beta deposition is one of the hallmark pathologies in both sporadic Alzheimer's disease and autosomal-dominant Alzheimer's disease, the latter of which is caused by mutations in genes involved in amyloid-beta processing. Despite amyloid-beta deposition being a centrepiece to both sporadic Alzheimer's disease and autosomal-dominant Alzheimer's disease, some differences between these Alzheimer's disease subtypes have been observed with respect to the spatial pattern of amyloid-beta. Previous work has shown that the spatial pattern of amyloid-beta in individuals spanning the sporadic Alzheimer's disease spectrum can be reproduced with high accuracy using an epidemic spreading model which simulates the diffusion of amyloid-beta across neuronal connections and is constrained by individual rates of amyloid-beta production and clearance. However, it has not been investigated whether amyloid-beta deposition in the rarer autosomal-dominant Alzheimer's disease can be modelled in the same way, and if so, how congruent the spreading patterns of amyloid-beta across sporadic Alzheimer's disease and autosomal-dominant Alzheimer's disease are. We leverage the epidemic spreading model as a data-driven approach to probe individual-level variation in the spreading patterns of amyloid-beta across three different large-scale imaging datasets (2 sporadic Alzheimer's disease, 1 autosomal-dominant Alzheimer's disease). We applied the epidemic spreading model separately to the Alzheimer's Disease Neuroimaging initiative (n = 737), the Open Access Series of Imaging Studies (n = 510) and the Dominantly Inherited Alzheimer's Network (n = 249), the latter two of which were processed using an identical pipeline. We assessed inter- and intra-individual model performance in each dataset separately and further identified the most likely subject-specific epicentre of amyloid-beta spread. Using epicentres defined in previous work in sporadic Alzheimer's disease, the epidemic spreading model provided moderate prediction of the regional pattern of amyloid-beta deposition across all three datasets. We further find that, whilst the most likely epicentre for most amyloid-beta-positive subjects overlaps with the default mode network, 13% of autosomal-dominant Alzheimer's disease individuals were best characterized by a striatal origin of amyloid-beta spread. These subjects were also distinguished by being younger than autosomal-dominant Alzheimer's disease subjects with a default mode network amyloid-beta origin, despite having a similar estimated age of symptom onset. Together, our results suggest that most autosomal-dominant Alzheimer's disease patients express amyloid-beta spreading patterns similar to those of sporadic Alzheimer's disease, but that there may be a subset of autosomal-dominant Alzheimer's disease patients with a separate, striatal phenotype.
Keywords: Alzheimer’s disease; amyloid beta; brain networks; diffusion models.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

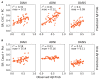
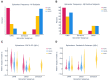
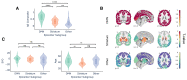
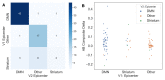
References
-
- Burns A, Iliffe S. Dementia. BMJ. 2009;338:b75–b75. - PubMed
-
- Shah H, Albanese E, Duggan C, et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016;15(12):1285–1294. - PubMed
-
- Hardy J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. - PubMed
-
- Goedert M, Masuda-Suzukake M, Falcon B. Like prions: The propagation of aggregated tau and α-synuclein in neurodegeneration. Brain. 2017;140(2):266–278. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
