CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells
- PMID: 35602937
- PMCID: PMC9118752
- DOI: 10.1016/j.isci.2022.104314
CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells
Abstract
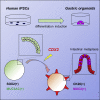
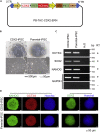
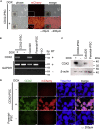
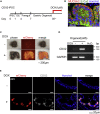
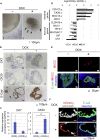
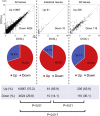
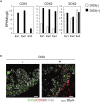
Intestinal metaplasia is related to gastric carcinogenesis. Previous studies have suggested the important role of CDX2 in intestinal metaplasia, and several reports have shown that the overexpression of CDX2 in mouse gastric mucosa caused intestinal metaplasia. However, no study has examined the induction of intestinal metaplasia using human gastric mucosa. In the present study, to produce an intestinal metaplasia model in human gastric mucosa in vitro, we differentiated human-induced pluripotent stem cells (hiPSC) to gastric organoids, followed by the overexpression of CDX2 using a tet-on system. The overexpression of CDX2 induced, although not completely, intestinal phenotypes and the enhanced expression of many, but not all, intestinal genes and previously reported intestinal metaplasia-related genes in the gastric organoids. This model can help clarify the mechanisms underlying intestinal metaplasia and carcinogenesis in human gastric mucosa and develop therapies to restitute precursor conditions of gastric cancer to normal mucosa.
Keywords: Biological sciences; Cell biology; Stem cells research.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice.Gut. 2004 Oct;53(10):1416-23. doi: 10.1136/gut.2003.032482. Gut. 2004. PMID: 15361487 Free PMC article.
-
Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice.Gastroenterology. 2002 Mar;122(3):689-96. doi: 10.1053/gast.2002.31902. Gastroenterology. 2002. PMID: 11875002
-
Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice.Cancer Res. 2004 Nov 1;64(21):7740-7. doi: 10.1158/0008-5472.CAN-04-1617. Cancer Res. 2004. PMID: 15520178
-
Premalignant conditions of gastric cancer.J Gastroenterol Hepatol. 2013 Jun;28(6):906-11. doi: 10.1111/jgh.12209. J Gastroenterol Hepatol. 2013. PMID: 23560829 Review.
-
Gastric intestinal metaplasia revisited: function and regulation of CDX2.Trends Mol Med. 2012 Sep;18(9):555-63. doi: 10.1016/j.molmed.2012.07.006. Epub 2012 Aug 4. Trends Mol Med. 2012. PMID: 22871898 Review.
Cited by
-
Revealing the role of metformin in gastric intestinal metaplasia treatment.Front Pharmacol. 2024 Jul 19;15:1340309. doi: 10.3389/fphar.2024.1340309. eCollection 2024. Front Pharmacol. 2024. PMID: 39101145 Free PMC article.
-
Severe induction of aberrant DNA methylation by nodular gastritis in adults.J Gastroenterol. 2024 Jun;59(6):442-456. doi: 10.1007/s00535-024-02094-y. Epub 2024 Mar 19. J Gastroenterol. 2024. PMID: 38499886
-
Revealing the pathogenesis of gastric intestinal metaplasia based on the mucosoid air-liquid interface.J Transl Med. 2024 May 17;22(1):468. doi: 10.1186/s12967-024-05276-7. J Transl Med. 2024. PMID: 38760813 Free PMC article.
-
Genetically engineered mouse models in gastric precancerous lesions research.World J Gastrointest Surg. 2025 Jul 27;17(7):107610. doi: 10.4240/wjgs.v17.i7.107610. World J Gastrointest Surg. 2025. PMID: 40740928 Free PMC article. Review.
-
Modeling gastric intestinal metaplasia in 3D organoids using nitrosoguanidine.J Mol Cell Biol. 2024 Dec 20;16(7):mjae030. doi: 10.1093/jmcb/mjae030. J Mol Cell Biol. 2024. PMID: 39153963 Free PMC article.
References
-
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M., et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

