JAM-A signals through the Hippo pathway to regulate intestinal epithelial proliferation
- PMID: 35602956
- PMCID: PMC9114518
- DOI: 10.1016/j.isci.2022.104316
JAM-A signals through the Hippo pathway to regulate intestinal epithelial proliferation
Abstract
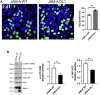
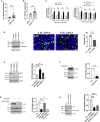
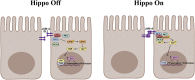
JAM-A is a tight-junction-associated protein that contributes to regulation of intestinal homeostasis. We report that JAM-A interacts with NF2 and LATS1, functioning as an initiator of the Hippo signaling pathway, well-known for regulation of proliferation. Consistent with these findings, we observed increased YAP activity in JAM-A-deficient intestinal epithelial cells (IEC). Furthermore, overexpression of a dimerization-deficient mutant, JAM-A-DL1, failed to initiate Hippo signaling, phenocopying JAM-A-deficient IEC, whereas overexpression of JAM-A-WT activated Hippo signaling and suppressed proliferation. Lastly, we identify EVI1, a transcription factor reported to promote cellular proliferation, as a contributor to the pro-proliferative phenotype in JAM-A-DL1 overexpressing IEC downstream of YAP. Collectively, our findings establish a new role for JAM-A as a cell-cell contact sensor, raising implications for understanding the contribution(s) of JAM-A to IEC proliferation in the mammalian epithelium.
Keywords: Biochemistry; Biological sciences; Cell biology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Barjesteh van Waalwijk van Doorn-Khosrovani S., Erpelinck C Fau - van Putten W.L.J., van Putten Wl Fau - Valk P.J.M., Valk Pj Fau - van der Poel-van de Luytgaarde S., van der Poel- van de Luytgaarde S Fau - Hack R., Hack R Fau - Slater R., Slater R., Fau - Smit E.M.E., Smit Em Fau - Beverloo H.B., Beverloo Hb Fau - Verhoef G., et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: a study of 319 de novo AML patients. Blood. 2003;101:837–845. - PubMed
-
- Brodowska K., Al-Moujahed A., Marmalidou A., Meyer Zu Horste M., Cichy J., Miller J.W., Gragoudas E., Vavvas D.G. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp. Eye Res. 2014;124:67–73. doi: 10.1016/j.exer.2014.04.011. - DOI - PMC - PubMed
-
- Cera M.R., Del Prete A., Vecchi A., Corada M., Martin-Padura I., Motoike T., Tonetti P., Bazzoni G., Vermi W., Gentili F., et al. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J. Clin. Invest. 2004;114:729–738. doi: 10.1172/JCI21231. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

