Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia
- PMID: 35603139
- PMCID: PMC9107685
- DOI: 10.1002/dad2.12285
Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia
Abstract
Introduction: We explored what combination of blood-based biomarkers (amyloid beta [Aβ]1-42/1-40, phosphorylated tau [p-tau]181, neurofilament light [NfL], glial fibrillary acidic protein [GFAP]) differentiates Alzheimer's disease (AD) dementia, frontotemporal dementia (FTD), and dementia with Lewy bodies (DLB).
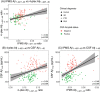
Methods: We measured the biomarkers with Simoa in two separate cohorts (n = 160 and n = 152). In one cohort, Aβ1-42/1-40 was also measured with mass spectrometry (MS). We assessed the differential diagnostic value of the markers, by logistic regression with Wald's backward selection.
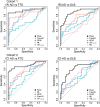
Results: MS and Simoa Aβ1-42/1-40 similarly differentiated AD from controls. The Simoa panel that optimally differentiated AD from FTD consisted of NfL and p-tau181 (area under the curve [AUC] = 0.94; cohort 1) or NfL, GFAP, and p-tau181 (AUC = 0.90; cohort 2). For AD from DLB, the panel consisted of NfL, p-tau181, and GFAP (AUC = 0.88; cohort 1), and only p-tau181 (AUC = 0.81; cohort 2).
Discussion: A combination of plasma p-tau181, NfL, and GFAP, but not Aβ1-42/1-40, might be useful to discriminate AD, FTD, and DLB.
Keywords: amyloid beta; blood biomarker; glial fibrillary acidic protein; neurofilament light; phosphorylated tau.
© 2022 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
ET, IV, JK, AA, and YP report no conflicts of interest. JV is an employee of ADx NeuroSciences. AB was an employee of Quanterix Corporation when the study was performed and holds shares in Quanterix. AL has received funding from stichting Dioraphte, Alzheimer Nederland, and ZonMW Memorabel (project #733050509). AL has performed contract research with Axovant, EIP Pharma, and Combinostics. All funding is paid to her institution. WF has performed contract research for Biogen MA Inc, and Boehringer Ingelheim. All funding is paid to her institution. WF has been an invited speaker at Boehringer Ingelheim, Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape), Springer Healthcare. All funding is paid to her institution. WF is consultant to Oxford Health Policy Forum CIC, Roche, and Biogen MA Inc. All funding is paid to her institution. WF participated in advisory boards of Biogen MA Inc and Roche. All funding is paid to her institution. WF was associate editor of Alzheimer, Research & Therapy in 2020/2021. WF is associate editor at Brain. All funding is paid to her institution. ES is an employee and shareholder of ADx NeuroSciences, Gent, Belgium. CH has a collaboration contract with Shimadzu European Innovation Center including a PhD thesis. CT has a collaboration contract with ADx NeuroSciences and Quanterix, performed contract research for or received grants from AC‐Immune, Axon Neurosciences, Biogen, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, PeopleBio, Roche, Toyama, Vivoryon. CT serves on editorial boards of Medidact Neurologi/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation, and is editor of a Neuromethods book for Springer. All funding is paid to her institution.
Figures


References
-
- WHO . Global action plan on the public health response to dementia 2017‐2025. World Health Organization; 2017. Licence: CC BY‐NC‐SA 3.0 IGO.
LinkOut - more resources
Full Text Sources
Miscellaneous
