PD1 Expression in EGFRvIII-Directed CAR T Cell Infusion Product for Glioblastoma Is Associated with Clinical Response
- PMID: 35603165
- PMCID: PMC9120664
- DOI: 10.3389/fimmu.2022.872756
PD1 Expression in EGFRvIII-Directed CAR T Cell Infusion Product for Glioblastoma Is Associated with Clinical Response
Abstract
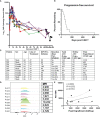
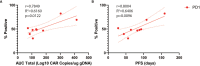
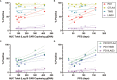
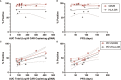
The epidermal growth factor receptor variant III (EGFRvIII) has been investigated as a therapeutic target for chimeric antigen receptor (CAR) T cell therapy in glioblastoma. Earlier research demonstrated that phenotypic and genotypic characteristics in T cells and CAR T product predicted therapeutic success in hematologic malignancies, to date no determinants for clinical response in solid tumors have been identified. We analyzed apheresis and infusion products from the first-in-human trial of EGFRvIII-directed CAR T for recurrent glioblastoma (NCT02209376) by flow cytometry. Clinical response was quantified via engraftment in peripheral circulation and progression-free survival (PFS), as determined by the time from CAR T infusion to first radiographic evidence of progression. The CD4+CAR T cell population in patient infusion products demonstrated PD1 expression which positively correlated with AUC engraftment and PFS. On immune checkpoint inhibitor analysis, CTLA-4, TIM3, and LAG3 did not exhibit significant associations with engraftment or PFS. The frequencies of PD1+GZMB+ and PD1+HLA-DR+ CAR T cells in the CD4+ infusion products were directly proportional to AUC and PFS. No significant associations were observed within the apheresis products. In summary, PD1 in CAR T infusion products predicted peripheral engraftment and PFS in recurrent glioblastoma.
Keywords: CAR T cells; GBM; PD-1; glioblastoma; immunotherapy.
Copyright © 2022 Tang, Tian, Yoder, Xu, Kulikovskaya, Gupta, Melenhorst, Lacey, O’Rourke and Binder.
Conflict of interest statement
DO’R receives laboratory support from Tmunity Therapeutics. DO’R and ZB are on patents relating to CAR T cell therapy for GBM. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Rivera AL, Pelloski CE, Gilbert MR, Colman H, de la Cruz C, Sulman EP, et al. . MGMT Promoter Methylation is Predictive of Response to Radiotherapy and Prognostic in the Absence of Adjuvant Alkylating Chemotherapy for Glioblastoma. Neuro Oncol (2010) 12(2):116–21. doi: 10.1093/neuonc/nop020 - DOI - PMC - PubMed
-
- O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. . A Single Dose of Peripherally Infused EGFRvIII-Directed CAR T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients With Recurrent Glioblastoma. Sci Transl Med (2017) 9(399):eaaa0984. doi: 10.1126/scitranslmed.aaa0984 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

