Autoantibody Landscape Revealed by Wet Protein Array: Sum of Autoantibody Levels Reflects Disease Status
- PMID: 35603173
- PMCID: PMC9114879
- DOI: 10.3389/fimmu.2022.893086
Autoantibody Landscape Revealed by Wet Protein Array: Sum of Autoantibody Levels Reflects Disease Status
Abstract
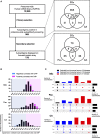
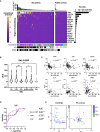
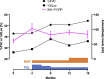
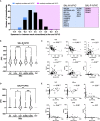
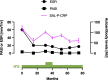
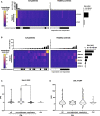
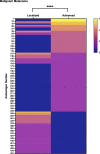
Autoantibodies are found in various pathological conditions such as autoimmune diseases, infectious diseases, and malignant tumors. However their clinical implications have not yet been fully elucidated. Herein, we conducted proteome-wide autoantibody screening and quantification with wet protein arrays consisting of proteins synthesized from proteome-wide human cDNA library (HuPEX) maintaining their three-dimensional structure. A total of 565 autoantibodies were identified from the sera of three representative inflammatory disorders (systemic sclerosis, psoriasis, and cutaneous arteritis). Each autoantibody level either positively or negatively correlated with serum levels of C-reactive protein, the best-recognized indicator of inflammation. In particular, we discovered total levels of a subset of autoantibodies correlates with the severity of clinical symptoms. From the sera of malignant melanoma, 488 autoantibodies were detected. Notably, patients with metastases had increased overall autoantibody production compared to those with tumors limiting to the primary site. Collectively, proteome-wide screening of autoantibodies using the in vitro proteome can reveal the "autoantibody landscape" of human subjects and may provide novel clinical biomarkers.
Keywords: autoantibody; autoimmunity; inflammatory disease; malignancy; protein array.
Copyright © 2022 Matsuda, Yoshizaki, Yamaguchi, Fukuda, Okumura, Ogawa, Ono, Norimatsu, Kotani, Hisamoto, Kawanabe, Kuzumi, Fukasawa, Ebata, Miyagawa, Yoshizaki-Ogawa, Goshima and Sato.
Conflict of interest statement
Authors KY, TO, KO, CO and NG were employed by ProteoBridge Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Arbuckle MR, McLain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. . Development of Autoantibodies Before the Clinical Onset of Systemic Lupus Erythematosus. N Engl J Med (2003) 349(16):1526–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

