Macrophage: A Cell With Many Faces and Functions in Tuberculosis
- PMID: 35603185
- PMCID: PMC9122124
- DOI: 10.3389/fimmu.2022.747799
Macrophage: A Cell With Many Faces and Functions in Tuberculosis
Abstract
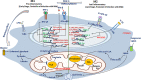
Mycobacterium tuberculosis (Mtb) is the causative agent of human tuberculosis (TB) which primarily infects the macrophages. Nearly a quarter of the world's population is infected latently by Mtb. Only around 5%-10% of those infected develop active TB disease, particularly during suppressed host immune conditions or comorbidity such as HIV, hinting toward the heterogeneity of Mtb infection. The aerosolized Mtb first reaches the lungs, and the resident alveolar macrophages (AMs) are among the first cells to encounter the Mtb infection. Evidence suggests that early clearance of Mtb infection is associated with robust innate immune responses in resident macrophages. In addition to lung-resident macrophage subsets, the recruited monocytes and monocyte-derived macrophages (MDMs) have been suggested to have a protective role during Mtb infection. Mtb, by virtue of its unique cell surface lipids and secreted protein effectors, can evade killing by the innate immune cells and preferentially establish a niche within the AMs. Continuous efforts to delineate the determinants of host defense mechanisms have brought to the center stage the crucial role of macrophage phenotypical variations for functional adaptations in TB. The morphological and functional heterogeneity and plasticity of the macrophages aid in confining the dissemination of Mtb. However, during a suppressed or hyperactivated immune state, the Mtb virulence factors can affect macrophage homeostasis which may skew to favor pathogen growth, causing active TB. This mini-review is aimed at summarizing the interplay of Mtb pathomechanisms in the macrophages and the implications of macrophage heterogeneity and plasticity during Mtb infection.
Keywords: Mycobacterium tuberculosis; innate immunity; macrophage heterogeneity; metabolic reprogramming; phenotype switching; trained immunity.
Copyright © 2022 Ahmad, Rani, Alam, Zarin, Pandey, Singh, Hasnain and Ehtesham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

