Annexin-A1 Tripeptide Attenuates Surgery-Induced Neuroinflammation and Memory Deficits Through Regulation the NLRP3 Inflammasome
- PMID: 35603196
- PMCID: PMC9120413
- DOI: 10.3389/fimmu.2022.856254
Annexin-A1 Tripeptide Attenuates Surgery-Induced Neuroinflammation and Memory Deficits Through Regulation the NLRP3 Inflammasome
Abstract
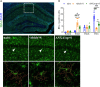
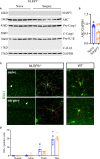
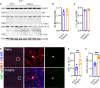
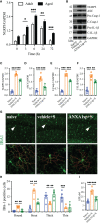
Neuroinflammation is a growing hallmark of perioperative neurocognitive disorders (PNDs), including delirium and longer-lasting cognitive deficits. We have developed a clinically relevant orthopedic mouse model to study the impact of a common surgical procedure on the vulnerable brain. The mechanism underlying PNDs remains unknown. Here we evaluated the impact of surgical trauma on the NLRP3 inflammasome signaling, including the expression of apoptosis-associated speck-like protein containing a CARD (ASC), caspase-1, and IL-1β in the hippocampus of C57BL6/J male mice, adult (3-months) and aged (>18-months). Surgery triggered ASC specks formation in CA1 hippocampal microglia, but without inducing significant morphological changes in NLRP3 and ASC knockout mice. Since no therapies are currently available to treat PNDs, we assessed the neuroprotective effects of a biomimetic peptide derived from the endogenous inflammation-ending molecule, Annexin-A1 (ANXA1). We found that this peptide (ANXA1sp) inhibited postoperative NLRP3 inflammasome activation and prevented microglial activation in the hippocampus, reducing PND-like memory deficits. Together our results reveal a previously under-recognized role of hippocampal ANXA1 and NLRP3 inflammasome dysregulation in triggering postoperative neuroinflammation, offering a new target for advancing treatment of PNDs through the resolution of inflammation.
Keywords: NLRP3 inflammasome; aging; annexin A1 derived peptide; microglia; postoperative cognition dysfunction.
Copyright © 2022 Zhang, Ma, Velagapudi, Barclay, Rodriguiz, Wetsel, Yang, Shinohara and Terrando.
Conflict of interest statement
ZZ and QM are co-inventors on patents filed through Duke University on the therapeutic use of ANXA1sp. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

