Advances in the Management of Primary Membranous Nephropathy and Rituximab-Refractory Membranous Nephropathy
- PMID: 35603210
- PMCID: PMC9114510
- DOI: 10.3389/fimmu.2022.859419
Advances in the Management of Primary Membranous Nephropathy and Rituximab-Refractory Membranous Nephropathy
Abstract
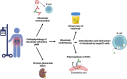
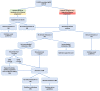
Primary membranous nephropathy (pMN) is an auto-immune disease characterized by auto-antibodies targeting podocyte antigens resulting in activation of complement and damage to the glomerular basement membrane. pMN is the most common cause of nephrotic syndrome in adults without diabetes. Despite a very heterogeneous course of the disease, the treatment of pMN has for many years been based on uniform management of all patients regardless of the severity of the disease. The identification of prognostic markers has radically changed the vision of pMN and allowed KDIGO guidelines to evolve in 2021 towards a more personalized management based on the assessment of the risk of progressive loss of kidney function. The recognition of pMN as an antibody-mediated autoimmune disease has rationalized the use immunosuppressive drugs such as rituximab. Rituximab is now a first line immunosuppressive therapy for patients with pMN with proven safety and efficacy achieving remission in 60-80% of patients. For the remaining 20-40% of patients, several mechanisms may explain rituximab resistance: (i) decreased rituximab bioavailability; (ii) immunization against rituximab; and (iii) chronic glomerular damage. The treatment of patients with rituximab-refractory pMN remains controversial and challenging. In this review, we provide an overview of recent advances in the management of pMN (according to the KDIGO 2021 guidelines), in the understanding of the pathophysiology of rituximab resistance, and in the management of rituximab-refractory pMN. We propose a treatment decision aid based on immunomonitoring to identify failures related to underdosing or immunization against rituximab to overcome treatment resistance.
Keywords: KDIGO (Kidney Disease: Improving Global Outcomes); PLA2R1 autoantibodies; autoimmunity; immunomonitoring; immunosuppressive therapy; membranous nephropathy; nephrotic syndrome; rituximab.
Copyright © 2022 Teisseyre, Cremoni, Boyer-Suavet, Ruetsch, Graça, Esnault, Brglez and Seitz-Polski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clin Pract Guideline Glomerulonephritis (2012), 86–97. doi:10.1038/kisup.2012.1 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

