Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test
- PMID: 35603292
- PMCID: PMC9053211
- DOI: 10.1038/s43856-022-00088-6
Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test
Abstract
Background: Detecting cancer at early stages significantly increases patient survival rates. Because lethal solid tumors often produce few symptoms before progressing to advanced, metastatic disease, diagnosis frequently occurs when surgical resection is no longer curative. One promising approach to detect early-stage, curable cancers uses biomarkers present in circulating extracellular vesicles (EVs). To explore the feasibility of this approach, we developed an EV-based blood biomarker classifier from EV protein profiles to detect stages I and II pancreatic, ovarian, and bladder cancer.
Methods: Utilizing an alternating current electrokinetics (ACE) platform to purify EVs from plasma, we use multi-marker EV-protein measurements to develop a machine learning algorithm that can discriminate cancer cases from controls. The ACE isolation method requires small sample volumes, and the streamlined process permits integration into high-throughput workflows.
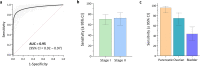
Results: In this case-control pilot study, comparison of 139 pathologically confirmed stage I and II cancer cases representing pancreatic, ovarian, or bladder patients against 184 control subjects yields an area under the curve (AUC) of 0.95 (95% CI: 0.92 to 0.97), with sensitivity of 71.2% (95% CI: 63.2 to 78.1) at 99.5% (97.0 to 99.9) specificity. Sensitivity is similar at both early stages [stage I: 70.5% (60.2 to 79.0) and stage II: 72.5% (59.1 to 82.9)]. Detection of stage I cancer reaches 95.5% in pancreatic, 74.4% in ovarian (73.1% in Stage IA) and 43.8% in bladder cancer.
Conclusions: This work demonstrates that an EV-based, multi-cancer test has potential clinical value for early cancer detection and warrants future expanded studies involving prospective cohorts with multi-year follow-up.
Keywords: Bladder cancer; Cancer screening; Ovarian cancer; Pancreatic cancer.
© The Author(s) 2022.
Conflict of interest statement
Competing interestsR. Krishnan, J.P.H., R.T., I.C., H.I.B., V.O., J.M.L., O.P., L.A., J.R.H., G.S., and D.S. are employees of Biological Dynamics. R. Krishnan is a co-founder and board member of Biological Dynamics. R. Krishnan is an inventor on patents held by the University of California San Diego and Biological Dynamics that covers aspects of the Verita™ platform used in this manuscript. The terms of these arrangements are being managed by the University of California–San Diego in accordance with its conflict-of-interest policies. R. Kurzrock receives research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, Bicara Therapeutics, Inc., Biological Dynamics, Neomed, Pfizer, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc. and ID by DNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch. R.E. receives research funding to his institution from Clovis Oncology, AVITA, Merck and AstraZenca, as well as consultant and/or speaker fees and/or advisory board from AstraZeneca, GSK/Tesaro, Seagen, Myriad, Merck, Eisai as well as the GOG Foundation. A.K. consultant/advisory board member for Abbott Molecular, Arquer, ArTara, Asieris, Astra Zeneca, BioClin Therapeutics, Biological Dynamics, BMS, Cepheid, Cold Genesys, Eisai, Engene, Inc., Ferring, FerGene, Imagin, Janssen, MDxHealth, Medac, Merck, Pfizer, Photocure, ProTara, Roviant, Seattle Genetics, Sessen Bio, Theralase, TMC Innovation, US Biotest. AM Kamat has received grant/research support from Adolor, BMS, FKD Industries, Heat Biologics, Merck, Photocure, SWOG/NIH, SPORE, AIBCCR. A.M.K. has patents for CyPRIT (Cytokine Predictors of Response to Intravesical Therapy) jointly with UT MD Anderson Cancer Center is a paid consultant of Biological Dynamics. S.M.L. is a co-founder of io9. N.J.S., S.M.L., P.B., and M.A. are members of the Biological Dynamics scientific advisory board. S.M.L. received principal investigator support from the UC San Diego Moores Cancer Center, Specialized Cancer Center Support Grant NIH/NCI P30CA023100, and SU2C-AACR-DT-25-17 Pancreatic Cancer Interception Dream Team award. A.M.L. and R.E. declare no competing interests. P.B. holds equity in CytoBay, Synergenz, and LungLifeAI, all cancer diagnostic or risk assessment enterprises.
Figures



References
-
- Muralidhar V, et al. Association between very small tumor size and decreased overall survival in node-positive pancreatic cancer. Ann. Surg. Oncol. 2018;25:4027–4034. - PubMed
-
- Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin. Oncol. Nurs. 2019;35:151–156. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA. 2021;71:7–33. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

