An olfactory self-test effectively screens for COVID-19
- PMID: 35603293
- PMCID: PMC9053292
- DOI: 10.1038/s43856-022-00095-7
An olfactory self-test effectively screens for COVID-19
Abstract
Background: Key to curtailing the COVID-19 pandemic are wide-scale screening strategies. An ideal screen is one that would not rely on transporting, distributing, and collecting physical specimens. Given the olfactory impairment associated with COVID-19, we developed a perceptual measure of olfaction that relies on smelling household odorants and rating them online.
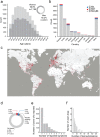
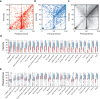
Methods: Each participant was instructed to select 5 household items, and rate their perceived odor pleasantness and intensity using an online visual analogue scale. We used this data to assign an olfactory perceptual fingerprint, a value that reflects the perceived difference between odorants. We tested the performance of this real-time tool in a total of 13,484 participants (462 COVID-19 positive) from 134 countries who provided 178,820 perceptual ratings of 60 different household odorants.
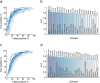
Results: We observe that olfactory ratings are indicative of COVID-19 status in a country, significantly correlating with national infection rates over time. More importantly, we observe indicative power at the individual level (79% sensitivity and 87% specificity). Critically, this olfactory screen remains effective in participants with COVID-19 but without symptoms, and in participants with symptoms but without COVID-19.
Conclusions: The current odorant-based olfactory screen adds a component to online symptom-checkers, to potentially provide an added first line of defense that can help fight disease progression at the population level. The data derived from this tool may allow better understanding of the link between COVID-19 and olfaction.
Keywords: Olfactory system; Signs and symptoms.
© The Author(s) 2022.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources

