Auditory deep sleep stimulation in older adults at home: a randomized crossover trial
- PMID: 35603302
- PMCID: PMC9053232
- DOI: 10.1038/s43856-022-00096-6
Auditory deep sleep stimulation in older adults at home: a randomized crossover trial
Abstract
Background: Auditory stimulation has emerged as a promising tool to enhance non-invasively sleep slow waves, deep sleep brain oscillations that are tightly linked to sleep restoration and are diminished with age. While auditory stimulation showed a beneficial effect in lab-based studies, it remains unclear whether this stimulation approach could translate to real-life settings.
Methods: We present a fully remote, randomized, cross-over trial in healthy adults aged 62-78 years (clinicaltrials.gov: NCT03420677). We assessed slow wave activity as the primary outcome and sleep architecture and daily functions, e.g., vigilance and mood as secondary outcomes, after a two-week mobile auditory slow wave stimulation period and a two-week Sham period, interleaved with a two-week washout period. Participants were randomized in terms of which intervention condition will take place first using a blocked design to guarantee balance. Participants and experimenters performing the assessments were blinded to the condition.
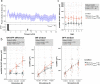
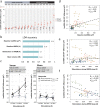
Results: Out of 33 enrolled and screened participants, we report data of 16 participants that received identical intervention. We demonstrate a robust and significant enhancement of slow wave activity on the group-level based on two different auditory stimulation approaches with minor effects on sleep architecture and daily functions. We further highlight the existence of pronounced inter- and intra-individual differences in the slow wave response to auditory stimulation and establish predictions thereof.
Conclusions: While slow wave enhancement in healthy older adults is possible in fully remote settings, pronounced inter-individual differences in the response to auditory stimulation exist. Novel personalization solutions are needed to address these differences and our findings will guide future designs to effectively deliver auditory sleep stimulations using wearable technology.
Keywords: Randomized controlled trials; Slow-wave sleep.
© The Author(s) 2022.
Conflict of interest statement
Competing interestsC.L. is a member of the Scientific Advisory Board of Emma Sleep GmbH, which is not related to this work. R.H. and W.K. are founders and shareholders of Tosoo AG that has licensed the technology used in this work. All others have no competing interests to declare.
Figures



References
-
- Ashford, R., Moore, P., Hu, B., Jackson, M. & Wan, J. Translational research and context in health monitoring systems. In 2010 International Conference on Complex, Intelligent and Software Intensive Systems 81–86 (IEEE, 2010).
-
- Woolf SH. The meaning of translational research and why it matters. Jama. 2008;299:211–213. - PubMed
-
- Sim I. Mobile devices and health. N. Engl. J. Med. 2019;381:956–968. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

