PAR2 promotes impaired glucose uptake and insulin resistance in NAFLD through GLUT2 and Akt interference
- PMID: 35603482
- PMCID: PMC9669194
- DOI: 10.1002/hep.32589
PAR2 promotes impaired glucose uptake and insulin resistance in NAFLD through GLUT2 and Akt interference
Abstract
Background and aims: Insulin resistance and poor glycemic control are key drivers of the development of NAFLD and have recently been shown to be associated with fibrosis progression in NASH. However, the underlying mechanisms involving dysfunctional glucose metabolism and relationship with NAFLD/NASH progression remain poorly understood. We set out to determine whether protease-activated receptor 2 (PAR2), a sensor of extracellular inflammatory and coagulation proteases, links NAFLD and NASH with liver glucose metabolism.
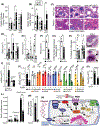
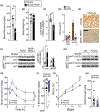
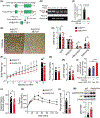
Approach and results: Here, we demonstrate that hepatic expression of PAR2 increases in patients and mice with diabetes and NAFLD/NASH. Mechanistic studies using whole-body and liver-specific PAR2-knockout mice reveal that hepatic PAR2 plays an unexpected role in suppressing glucose internalization, glycogen storage, and insulin signaling through a bifurcating Gq -dependent mechanism. PAR2 activation downregulates the major glucose transporter of liver, GLUT2, through Gq -MAPK-FoxA3 and inhibits insulin-Akt signaling through Gq -calcium-CaMKK2 pathways. Therapeutic dosing with a liver-homing pepducin, PZ-235, blocked PAR2-Gq signaling and afforded significant improvements in glycemic indices and HbA1c levels in severely diabetic mice.
Conclusions: This work provides evidence that PAR2 is a major regulator of liver glucose homeostasis and a potential target for the treatment of diabetes and NASH.
© 2022 American Association for the Study of Liver Diseases.
Conflict of interest statement
CONFLICT OF INTEREST
Athan Kuliopulos is the scientific cofounder of Oasis Pharmaceuticals. Lidija Covic is the scientific cofounder of Oasis Pharmaceuticals.
Figures




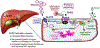
Comment in
-
Letter to the Editor: The pathological impact of PAR2 on hepatic insulin resistance.Hepatology. 2023 May 1;77(5):E109-E110. doi: 10.1097/HEP.0000000000000284. Epub 2023 Jan 3. Hepatology. 2023. PMID: 36869844 No abstract available.
References
-
- Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

