Wnt family member 1 (Wnt1) overexpression-induced M2 polarization of microglia alleviates inflammation-sensitized neonatal brain injuries
- PMID: 35603707
- PMCID: PMC9275958
- DOI: 10.1080/21655979.2022.2074767
Wnt family member 1 (Wnt1) overexpression-induced M2 polarization of microglia alleviates inflammation-sensitized neonatal brain injuries
Abstract
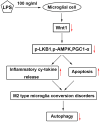
Intrauterine infection induces inflammation-mediated microglial activation and brain injury. This study aimed to explore the regulatory mechanism of Wnt family member 1 (Wnt1) in intrauterine infection-mediated microglial polarization. The cell counting kit-8 (CCK-8) assay was used to determine the viability of microglia, and cytokine expression levels were determined using enzyme linked immunosorbent assay (ELISA) kits and real-time quantitative PCR (RT-qPCR). The number of CD206+ and CD16/32+ cells was determined by flow cytometry. Wnt1 expression was analyzed using western blotting and immunofluorescence. Moreover, an in vivo assay was performed to verify the role of WNT1 in inflammation-sensitized brain injury in newborn mice. Lipopolysaccharide (LPS) exposure resulted in a decrease in microglial cell viability while increasing the expression levels of inflammatory cytokines (TNF-α, IL-6, and IL-1β), simultaneously promoting M1-type microglial conversion. However, these effects were rescued by overexpression of Wnt1, which was expressed less in microglia exposed to LPS in vitro and in vivo. Here, we found that Wnt1 activated the LKB1-AMPK pathway, and the inhibition of LKB1 attenuated the rescue effects of Wnt1. In addition, LPS exposure reduced the autophagy of microglia, and Wnt1 overexpression enhanced the autophagy, but this effect was reversed by treatment with an LKB1 inhibitor. Wnt1 activated LKB1 to suppress inflammation-mediated activation of microglia, promote M2-type microglia conversion via the AMPK pathway, and alleviate inflammation-sensitized neonatal brain injuries. This provides a potential avenue for the treatment of neonatal brain injuries.
Keywords: AMPK; LKB1; Microglia polarization; autophagy; wnt1.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures







References
-
- Fu CH, Zhang BH, Fang CZ, et al. Long non-coding RNA CRNDE deteriorates intrauterine infection-induced neonatal brain injury. Mol Cell Probes. 2020;52:101565. - PubMed
-
- Yuan TM, Sun Y, Zhan CY, et al. Intrauterine infection/inflammation and perinatal brain damage: role of glial cells and Toll-like receptor signaling. J Neuroimmunol. 2010;229(1–2):16–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
