Zinc-Catalyzed Enantioselective [3+2] Cycloaddition of Azomethine Ylides Using Planar Chiral [2.2]Paracyclophane-Imidazoline N,O-ligands
- PMID: 35603757
- PMCID: PMC9543521
- DOI: 10.1002/anie.202205516
Zinc-Catalyzed Enantioselective [3+2] Cycloaddition of Azomethine Ylides Using Planar Chiral [2.2]Paracyclophane-Imidazoline N,O-ligands
Abstract
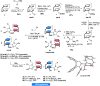
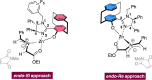
We present a facile synthetic route toward a novel series of imidazolinyl-[2.2]paracyclophanol (UCD-Imphanol) ligands possessing central and planar chirality. Both sets of diastereomeric ligands were successfully purified by column chromatography. The preliminary application of this family of ligands showed excellent activities in the asymmetric Zn-catalyzed azomethine ylide cycloaddition. Enantioenriched pyrrolidines, in a substrate scope of 20 examples, were accessed in high levels of endo/exo ratios (up to >99/1) and enantioselectivities (up to >99 % ee) with excellent yields (up to 99 %) by using (S,S,SP )-UCD-Imphanol/(S,S,RP )-UCD-Imphanol, respectively.
Keywords: Asymmetric Catalysis; Cycloaddition; Cyclophanes; Planar Chiral; Zinc Catalysis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- None
-
- Gibson S. E., Knight J. D., Org. Biomol. Chem. 2003, 1, 1256–1269; - PubMed
-
- Rozenberg V., Sergeeva E., Hopf H., Modern Cyclophane Chemistry, Wiley-VCH, Weinheim, 2004, pp. 435–462;
-
- Hopf H., Angew. Chem. Int. Ed. 2008, 47, 9808–9812; - PubMed
- Angew. Chem. 2008, 120, 9954–9958;
-
- Elacqua E., MacGillivray L. R., Eur. J. Org. Chem. 2010, 6883–6894;
Grants and funding
LinkOut - more resources
Full Text Sources

