Cabozantinib-nivolumab sequence in metastatic renal cell carcinoma: The CABIR study
- PMID: 35603906
- PMCID: PMC9541795
- DOI: 10.1002/ijc.34126
Cabozantinib-nivolumab sequence in metastatic renal cell carcinoma: The CABIR study
Abstract
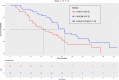
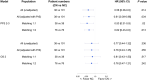
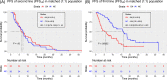
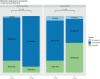
Nivolumab and cabozantinib are approved agents in mRCC patients after sunitinib/pazopanib (TKI) failure. However, the optimal sequence, cabozantinib then nivolumab (CN) or nivolumab then cabozantinib (NC), is still unknown. The CABIR study aimed to identify the optimal sequence between CN and NC after frontline VEGFR-TKI. In this multicenter retrospective study, we collected data from mRCC pts receiving CN or NC, after frontline VEGFR-TKI. A propensity score (PrS) was calculated to manage bias selection, and sequence comparisons were carried out with a cox model on a matched sample 1:1. The primary endpoint was progression-free survival (PFS) from the start of second line to progression in third line (PFS2-3 ). Key secondary endpoints included overall survival from second line (OS2 ). Out of 139 included mRCC patients, 38 (27%) and 101 (73%) received CN and NC, respectively. Overlap in PrS allowed 1:1 matching for each CN pts, with characteristics well balanced. For both PFS2-3 and OS2 , NC sequence was superior to CN (PFS2-3 : HR = 0.58 [0.34-0.98], P = .043; OS2 : 0.66 [0.42-1.05], P = .080). Superior PFS2-3 was in patients treated between 6 and 18 months with prior VEGFR-TKI (P = .019) and was driven by a higher PFSL3 with cabozantinib when given after nivolumab (P < .001). The CABIR study shows a prolonged PFS of the NC sequence compared to CN in mRCC after first line VEGFR-TKI failure. The data suggest that cabozantinib may be more effective than nivolumab in the third-line setting, possibly related to an ability of cabozantinib to overcome resistance to PD-1 blockade.
Keywords: cabozantinib; immunotherapy; matching-adjusted study; nivolumab; renal cell carcinoma; tyrosine kinase inhibitor.
© 2022 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Yann‐Alexandre Vano received consultancy fees from BMS, MSD, Ipsen, Merck, Pfizer, Novartis. Gwenaelle Gravis: consultancy fees were received by her institution from BMS, Pfizer, MSD, Alliance Merck‐Pfizer; fees received by her institution for coordinating PI role from BMS. Denis Maillet received consultancy fees from BMS, Ipsen, Pfizer, MSD. Delphine Borchiellini: received consultancy fees from Astellas, AstraZeneca, Bayer, BMS, Ipsen, Janssen, Merck, MSD, Pfizer; clinical research funding (institution) from: Astellas, AstraZeneca, Bayer, BMS, Exelixis, Infinity, Janssen, MSD, Pfizer, Roche, Taiho Oncology. Philippe Barthelemy received consultancy fees from BMS, Ipsen Pfizer, Merck MSD Novartis. Raffaele Ratta received consultancy fees from BMS, MSD, Ipsen, Merck, Pfizer. Sheik Emambux received consultancy fees from BMS and IPSEN. Stéphane Oudard received consultancy fees from Merck, Novartis, Pfizer, BMS, Ipsen. Mostefa Bennamoun, Letuan Phan, Iphigénie Korakis, Friederike Schlürmann, Nadine Houede, Delphine Topart, Thomas Ryckewaert, Ali Hasbini, Sophie Hans, Sandra Cournier, Elena Braychenko, Réza‐Thierry Elaidi: none.
Figures

References
-
- Gurram S, Al Harthy M, Ball MW. The changing landscape of systemic therapy in metastatic renal cell carcinoma: an update. Discov Med. 2020;29:191‐199. - PubMed
-
- Bedke J, Albiges L, Capitanio U, et al. The 2021 updated European Association of Urology guidelines on renal cell carcinoma: immune checkpoint inhibitor‐based combination therapies for treatment‐naive metastatic clear‐cell renal cell carcinoma are standard of care. Eur Urol. 2021;80:393‐397. doi:10.1016/j.eururo.2021.04.042 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

