Detectable Duration of Viable SARS-CoV-2, Total and Subgenomic SARS-CoV-2 RNA in Noncritically Ill COVID-19 Patients: a Prospective Cohort Study
- PMID: 35604133
- PMCID: PMC9241878
- DOI: 10.1128/spectrum.00503-22
Detectable Duration of Viable SARS-CoV-2, Total and Subgenomic SARS-CoV-2 RNA in Noncritically Ill COVID-19 Patients: a Prospective Cohort Study
Abstract
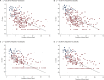
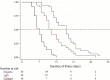
Determination of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity is important in guiding the infection control and differentiating between reinfection and persistent viral RNA. Although viral culture is the gold standard to determine viral infectivity, the method is not practical. We studied the kinetics of SARS-CoV-2 total RNAs and subgenomic RNAs (sgRNAs) and their potential role as surrogate markers of viral infectivity. The kinetics of SARS-CoV-2 sgRNAs compared to those of the culture and total RNA shedding in a prospective cohort of patients diagnosed with coronavirus disease 2019 (COVID-19) were investigated. A total of 260 nasopharyngeal swabs from 36 patients were collected every other day after entering the study until the day of viral total RNA clearance, as measured by reverse transcription PCR (RT-PCR). Time to cessation of viral shedding was in order from shortest to longest: by viral culture, sgRNA RT-PCR, and total RNA RT-PCR. The median time (interquartile range) to negativity of viral culture, subgenomic N transcript, and N gene were 7 (5 to 9), 11 (9 to 16), and 18 (13 to 21) days, respectively (P < 0.001). Further analysis identified the receipt of steroid as the factors associated with longer duration of viral infectivity (hazard ratio, 3.28; 95% confidence interval, 1.02 to 10.61; P = 0.047). We propose the potential role of the detection of SARS-CoV-2 subgenomic RNA as the surrogate marker of viral infectivity. Patients with negative subgenomic N RNA RT-PCR could be considered for ending isolation. IMPORTANCE Our study, combined with existing evidence, suggests the feasibility of the use of subgenomic RNA RT-PCR as a surrogate marker for SARS-CoV-2 infectivity. The kinetics of SARS-CoV-2 subgenomic RNA should be further investigated in immunocompromised patients.
Keywords: COVID-19; SARS-CoV-2; infectivity; subgenomic RNA; viral shedding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
SARS-CoV-2 Virus Culture, Genomic and Subgenomic RNA Load, and Rapid Antigen Test in Experimentally Infected Syrian Hamsters.J Virol. 2022 Sep 28;96(18):e0103422. doi: 10.1128/jvi.01034-22. Epub 2022 Aug 30. J Virol. 2022. PMID: 36040179 Free PMC article.
-
Profiling of SARS-CoV-2 Subgenomic RNAs in Clinical Specimens.Microbiol Spectr. 2022 Apr 27;10(2):e0018222. doi: 10.1128/spectrum.00182-22. Epub 2022 Mar 21. Microbiol Spectr. 2022. PMID: 35311586 Free PMC article.
-
Viral Culture Confirmed SARS-CoV-2 Subgenomic RNA Value as a Good Surrogate Marker of Infectivity.J Clin Microbiol. 2022 Jan 19;60(1):e0160921. doi: 10.1128/JCM.01609-21. Epub 2021 Oct 20. J Clin Microbiol. 2022. PMID: 34669457 Free PMC article.
-
SARS-CoV-2 Subgenomic RNAs: Characterization, Utility, and Perspectives.Viruses. 2021 Sep 24;13(10):1923. doi: 10.3390/v13101923. Viruses. 2021. PMID: 34696353 Free PMC article. Review.
-
Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): Review of current literature.Infect Control Hosp Epidemiol. 2021 Jun;42(6):659-668. doi: 10.1017/ice.2020.1273. Epub 2020 Oct 20. Infect Control Hosp Epidemiol. 2021. PMID: 33077007 Free PMC article. Review.
Cited by
-
Quantitative SARS-CoV-2 subgenomic RNA as a surrogate marker for viral infectivity: Comparison between culture isolation and direct sgRNA quantification.PLoS One. 2023 Sep 1;18(9):e0291120. doi: 10.1371/journal.pone.0291120. eCollection 2023. PLoS One. 2023. PMID: 37656746 Free PMC article.
-
Successful lung transplantation using an allograft from a COVID-19-recovered donor: a potential role for subgenomic RNA to guide organ utilization.Am J Transplant. 2023 Jan;23(1):101-107. doi: 10.1016/j.ajt.2022.09.001. Epub 2023 Jan 11. Am J Transplant. 2023. PMID: 36695611 Free PMC article.
-
Risk of transmission of COVID-19 from healthcare workers returning to work after a 5-day isolation, and kinetics of shedding of viable SARS-CoV-2 variant B.1.1.529 (Omicron).J Hosp Infect. 2023 Jan;131:228-233. doi: 10.1016/j.jhin.2022.11.012. Epub 2022 Nov 29. J Hosp Infect. 2023. PMID: 36460176 Free PMC article.
-
SARS-CoV-2 Virus Culture, Genomic and Subgenomic RNA Load, and Rapid Antigen Test in Experimentally Infected Syrian Hamsters.J Virol. 2022 Sep 28;96(18):e0103422. doi: 10.1128/jvi.01034-22. Epub 2022 Aug 30. J Virol. 2022. PMID: 36040179 Free PMC article.
-
Persistence of SARS-CoV-2 RNA shedding and infectivity in immunized population: Prospective study along different epidemiological periods in Argentina.PLoS One. 2023 May 17;18(5):e0285704. doi: 10.1371/journal.pone.0285704. eCollection 2023. PLoS One. 2023. PMID: 37196044 Free PMC article.
References
-
- World Health Organization. 2020. WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020. World Health Organization, Geneva, Switzerland. https://www.who.int/director-general/speeches/detail/who-director-genera.... Accessed November 10, 2021.
-
- Hanson KE, Caliendo AM, Arias CA, Hayden MK, Englund JA, Lee MJ, Loeb M, Patel R, El Alayli A, Altayar O, Patel P, Falck-Ytter Y, Lavergne V, Morgan RL, Murad MH, Sultan S, Bhimraj A, Mustafa RA. 2021. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Clin Infect Dis ciab048. doi:10.1093/cid/ciab048. - DOI - PMC - PubMed
-
- Tarhini H, Recoing A, Bridier-Nahmias A, Rahi M, Lambert C, Martres P, Lucet JC, Rioux C, Bouzid D, Lebourgeois S, Descamps D, Yazdanpanah Y, Le Hingrat Q, Lescure FX, Visseaux B. 2021. Long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis 223:1522–1527. doi:10.1093/infdis/jiab075. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

