Characterization of Glucokinases from Pathogenic Free-Living Amoebae
- PMID: 35604214
- PMCID: PMC9211422
- DOI: 10.1128/aac.02373-21
Characterization of Glucokinases from Pathogenic Free-Living Amoebae
Abstract
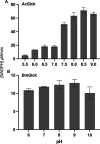
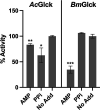
Infection with pathogenic free-living amoebae, including Naegleria fowleri, Acanthamoeba spp., and Balamuthia mandrillaris, can lead to life-threatening illnesses, primarily because of catastrophic central nervous system involvement. Efficacious treatment options for these infections are lacking, and the mortality rate due to infection is high. Previously, we evaluated the N. fowleri glucokinase (NfGlck) as a potential target for therapeutic intervention, as glucose metabolism is critical for in vitro viability. Here, we extended these studies to the glucokinases from two other pathogenic free-living amoebae, including Acanthamoeba castellanii (AcGlck) and B. mandrillaris (BmGlck). While these enzymes are similar (49.3% identical at the amino acid level), they have distinct kinetic properties that distinguish them from each other. For ATP, AcGlck and BmGlck have apparent Km values of 472.5 and 41.0 μM, while Homo sapiens Glck (HsGlck) has a value of 310 μM. Both parasite enzymes also have a higher apparent affinity for glucose than the human counterpart, with apparent Km values of 45.9 μM (AcGlck) and 124 μM (BmGlck) compared to ~8 mM for HsGlck. Additionally, AcGlck and BmGlck differ from each other and other Glcks in their sensitivity to small molecule inhibitors, suggesting that inhibitors with pan-amoebic activity could be challenging to generate.
Keywords: Acanthamoeba castellanii; Balamuthia mandrillaris; Naegleria fowleri; free-living amoeba; glucokinase; glycolysis; inhibitors; pentose phosphate pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Milanes JE, Suryadi J, Abendroth J, Van Voorhis WC, Barrett KF, Dranow DM, Phan IQ, Patrick SL, Rozema SD, Khalifa MM, Golden JE, Morris JC. 2019. Enzymatic and structural characterization of the Naegleria fowleri glucokinase. Antimicrob Agents Chemother 63:e02410-18. 10.1128/AAC.02410-18. - DOI - PMC - PubMed
-
- Lorenzo-Morales J, Kliescikova J, Martinez-Carretero E, De Pablos LM, Profotova B, Nohynkova E, Osuna A, Valladares B. 2008. Glycogen phosphorylase in Acanthamoeba spp.: determining the role of the enzyme during the encystment process using RNA interference. Eukaryot Cell 7:509–517. 10.1128/EC.00316-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

