Contractile force assessment methods for in vitro skeletal muscle tissues
- PMID: 35604384
- PMCID: PMC9126583
- DOI: 10.7554/eLife.77204
Contractile force assessment methods for in vitro skeletal muscle tissues
Abstract
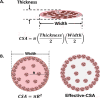
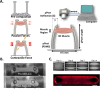
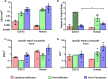
Over the last few years, there has been growing interest in measuring the contractile force (CF) of engineered muscle tissues to evaluate their functionality. However, there are still no standards available for selecting the most suitable experimental platform, measuring system, culture protocol, or stimulation patterns. Consequently, the high variability of published data hinders any comparison between different studies. We have identified that cantilever deflection, post deflection, and force transducers are the most commonly used configurations for CF assessment in 2D and 3D models. Additionally, we have discussed the most relevant emerging technologies that would greatly complement CF evaluation with intracellular and localized analysis. This review provides a comprehensive analysis of the most significant advances in CF evaluation and its critical parameters. In order to compare contractile performance across experimental platforms, we have used the specific force (sF, kN/m2), CF normalized to the calculated cross-sectional area (CSA). However, this parameter presents a high variability throughout the different studies, which indicates the need to identify additional parameters and complementary analysis suitable for proper comparison. We propose that future contractility studies in skeletal muscle constructs report detailed information about construct size, contractile area, maturity level, sarcomere length, and, ideally, the tetanus-to-twitch ratio. These studies will hopefully shed light on the relative impact of these variables on muscle force performance of engineered muscle constructs. Prospective advances in muscle tissue engineering, particularly in muscle disease models, will require a joint effort to develop standardized methodologies for assessing CF of engineered muscle tissues.
Keywords: contractile force; neuroscience; skeletal muscle; stimulation; tissue engineering.
© 2022, Vesga-Castro et al.
Conflict of interest statement
CV, JA, AV, JP No competing interests declared
Figures



References
-
- Afshar ME, Abraha HY, Bakooshli MA, Davoudi S, Thavandiran N, Tung K, Ahn H, Ginsberg HJ, Zandstra PW, Gilbert PM. A 96-well culture platform enables longitudinal analyses of engineered human skeletal muscle microtissue strength. Scientific Reports. 2020;10:1–16. doi: 10.1038/s41598-020-62837-8. - DOI - PMC - PubMed
-
- Afshar Bakooshli M, Lippmann ES, Mulcahy B, Iyer N, Nguyen CT, Tung K, Stewart BA, van den Dorpel H, Fuehrmann T, Shoichet M, Bigot A, Pegoraro E, Ahn H, Ginsberg H, Zhen M, Ashton RS, Gilbert PM. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. eLife. 2019;8:e44530. doi: 10.7554/eLife.44530. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

