In vitro-in silico-based prediction of inter-individual and inter-ethnic variations in the dose-dependent cardiotoxicity of R- and S-methadone in humans
- PMID: 35604418
- PMCID: PMC9217890
- DOI: 10.1007/s00204-022-03309-y
In vitro-in silico-based prediction of inter-individual and inter-ethnic variations in the dose-dependent cardiotoxicity of R- and S-methadone in humans
Abstract
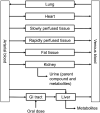
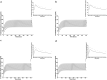
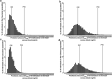
New approach methodologies predicting human cardiotoxicity are of interest to support or even replace in vivo-based drug safety testing. The present study presents an in vitro-in silico approach to predict the effect of inter-individual and inter-ethnic kinetic variations in the cardiotoxicity of R- and S-methadone in the Caucasian and the Chinese population. In vitro cardiotoxicity data, and metabolic data obtained from two approaches, using either individual human liver microsomes or recombinant cytochrome P450 enzymes (rCYPs), were integrated with physiologically based kinetic (PBK) models and Monte Carlo simulations to predict inter-individual and inter-ethnic variations in methadone-induced cardiotoxicity. Chemical specific adjustment factors were defined and used to derive dose-response curves for the sensitive individuals. Our simulations indicated that Chinese are more sensitive towards methadone-induced cardiotoxicity with Margin of Safety values being generally two-fold lower than those for Caucasians for both methadone enantiomers. Individual PBK models using microsomes and PBK models using rCYPs combined with Monte Carlo simulations predicted similar inter-individual and inter-ethnic variations in methadone-induced cardiotoxicity. The present study illustrates how inter-individual and inter-ethnic variations in cardiotoxicity can be predicted by combining in vitro toxicity and metabolic data, PBK modelling and Monte Carlo simulations. The novel methodology can be used to enhance cardiac safety evaluations and risk assessment of chemicals.
Keywords: Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM); Inter-individual differences; Methadone; Monte Carlo simulation; New approach methodologies (NAM); Physiologically based kinetic (PBK) modelling; Quantitative in vitro to in vivo extrapolation (QIVIVE).
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures


References
-
- Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human micro-somal protein and hepatocellularity per gram of liver. Curr Drug Metab. 2007;8:33–45. doi: 10.2174/138920007779315053. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

